 |
 |
 |

September / October 2007

Reducing Complexity:
A Strategic Approach to Optimizing the Medication Use Process for All Medications
Tim Vanderveen, PharmD, MS; Stephen R. Lewis, MD;

Sherri Almeida, DrPH, MSN, RN, CEN, FAEN
The 2006 Institute of Medicine (IOM) report, Preventing Medication Errors, found that patients experience more than 400,000 adverse drug events (ADEs) annually (IOM, 2006). Each ADE results in an estimated $8,750 in extra hospital costs, a total cost of $3.5 billion annually (2006 dollars). At least a quarter of all medication-related injuries are preventable. The report calls for hospitals to take action to improve medication safety; specifically, to reduce medication errors and ADEs. According to the IOM, a major cause of medication errors is the complexity of the medication-use process (IOM, 2006).
Complexity leads to variation in practice, opportunities for medication errors, and increased risk of patient harm. Reducing complexity is key to improving both safety and productivity. Human engineering and complexity theory teach that hospitals need to:
- Simplify

- Standardize

- Automate

- Integrate
Critical Challenges
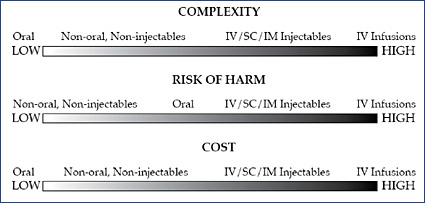
Oral solids and other non-intravenous drugs are associated with the greatest number of medication orders. Intravenous (IV) medications present the greatest complexity, risk of harm (Williams & Maddox, 2005), and cost (Figure 1).
For an oral solid or liquid, an IV, subcutaneous (SC), intramuscular (IM), or IV injection, or ophthalmics and other topical medications, administration of a dose is a one-point-in-time "event." However, for most IV medication infusions, administration is a "process" that occurs over time and can involve bolus doses and numerous dosage adjustments, which greatly increase complexity.
Many of the highest-risk drugs, e.g., heparin, insulin, and narcotics, are delivered by IV infusion (Williams & Maddox, 2005). This route of administration often results in the most serious adverse outcomes from medication errors (Hicks, et al., 2003). Of the most serious potential ADEs, 61% are IV-related (Bates, 2001). Data have shown that for every 1000 patient days, 1.1 potentially life-threatening and an additional 1.5 potentially significant IV medication programming errors were averted by IV safety systems (Cardinal Health, 2005).
IV medications are also costly. Drug costs represent the great majority of pharmacy budgets, and IV medications are recognized as being the most costly. These costs are projected to increase 5% to 7% annually (Hoffman, et al., 2006).
Safety on the patient care unit and at the point of care is critical. Numerous factors increase complexity (Tables 1 and 2). In terms of medication use, nurses need to assess the patient and review medication orders and other pertinent information for that particular patient, then efficiently access the correct medication and prepare it. Before administering medication, they have to positively identify the patient, medication, route, and time to verify the "five rights." Finally, they administer the medication, document administration, and monitor patient response. Every step presents opportunities for errors.
Safety Is a System Property
Adverse medical events are usually the result of systems problems. Just as aviation safety improved only after industry leaders adopted this tenet, safety in medicine will not improve unless its complex systems are redesigned (Meisel, 2006). Ultimately, the only way to improve safety is to create a culture of safety that strives to improve the medication administration system and to reduce the inherent complexity of medication management and use. Technology is used to help prevent errors and enable people to do the right thing. The IOM recommends that healthcare organizations adopt practices and technologies that can reduce medication errors, use well-designed technologies, and create interoperability among systems (IOM, 2006).
According to the IOM, all healthcare provider groups should seek to be high-reliability organizations (HROs) preoccupied with the possibility of failure (IOM, 2006). HROs know that every safe system has built-in, redundant checks and fail-safes. The medication-use process is already designed for this: multiple check points exist to check for accuracy and track medications throughout the process. The goal is to seamlessly integrate all aspects of the system.

As illustrated in Reason's well-known "Swiss cheese" model, a system must have multiple "slices" to reliably prevent errors. If a nurse gets to the bedside before discovering a problem, he or she then has to backtrack, and the system has already exposed the patient to a 98% risk that a potential ADE may not be intercepted (Leape et al., 1995).
A high-reliability system pushes checks upstream by controlling medications throughout the entire medication-use process. The use of automation within the pharmacy to order, receive, store, and dispense medications provides redundant safety checks and helps improve productivity, detect narcotic diversion, and reduce waste and shrinkage.
Automated dispensing machines (ADMs) provide a mechanism to integrate and standardize the pharmacy dispensing processes with both the nursing drug administration processes and the reordering processes from the pharmaceutical wholesaler. At the point of care, wireless barcode verification and smart infusion systems simplify, standardize, automate, integrate, and add a layer of safety to the actual administration of the medication. Effective medication management safeguards all types of medications (Figure 2) and integrates all processes of medication use (Figure 3).
System Integration
The following descriptions suggest how an integrated system would make the medication-use process, including drug administration at the point of care, both simpler and safer for all medications (Figures 2 and 3, Table 3).
An essential first step is to simplify and standardize the formulary. This reduces complexity throughout the medication-use process.
In the absence of computerized prescriber order entry (CPOE), electronic order management incorporating order scanning improves accuracy and speeds turnaround time. Once an order is approved by pharmacy, a decentralized pharmacy system using ADMs can allow immediate access to approximately 90% of all medications (Maddox, 2005), improving turnaround and first-dose processing.
A critical step is to determine how the hospital will routinely provide barcode-labeled medications. Barcode scanning technology can be used across the medication-use process to improve accuracy, track medications, streamline workflow, and provide data for quality improvement initiatives.
Wireless barcode technology helps promote accuracy in selecting the correct medication in the pharmacy (Miviglia, et al., 2007) and in stocking and restocking the ADMs on the patient care unit. By providing a complete chain-of-custody for medications during distribution to the patient care unit, hospitals can ensure that the right medication is getting to the right station and the right pocket in the station.
At the point of care, a wireless handheld device or a computer with advanced wireless barcode scanning technology can be used to positively identify the patient, verify medication orders and check "the five rights" for all medication. However, safety concerns go beyond "the five rights" to also include the patient's condition, i.e., has the patient's condition significantly improved or deteriorated since the current orders were written or medication last administered? For titration of high-risk IV drugs such as heparin and insulin, what should be the next dose administered?
Barcode technology alone is not sufficient to ensure the safety of continuous IV infusions. Unless the infusion and barcode technology are fully integrated, accurate device programming cannot be confirmed. A continuous IV infusion often spans multiple nursing shifts, with multiple clinicians making periodic dosage adjustments based on laboratory results, protocols or verbal orders that may not be included in the barcode system. These scenarios increase the possibility of programming errors. Advanced infusion safety systems help prevent IV medication errors by providing drug libraries, best practices, and alerts when pump programming is outside of hospital-established limits (Vanderveen, 2006).
Modular infusion safety systems provide a common platform for all types of infusionslarge volume parenterals, syringe, and PCA. Some systems combine IV barcoding and non-invasive respiratory monitoring. A common interface streamlines clinical workflow, simplifies staff training, reduces programming complexity, and increases ease of use (Maddox, et al., 2006). Information available from combined dosing and respiratory monitoring trend data, particularly for end-tidal carbon dioxide (EtCO2), helps reduce the risk of PCA-related respiratory depression (Maddox, et al., 2006).
Barcode-enabled bedside point-of-care systems using positive patient identification (PPID) that are care-integration and decision-support tools may provide a higher level of safety by integrating data from vital sign monitors, laboratory databases, ventilators, pulse oximeters, clinical decision-support and other tools. In addition to improving safety for complex IV medications, these systems may also support and integrate data from care processes involving total parenteral nutrition (TPN), blood transfusions, breast milk, patient charting, viewing, and charge capture. Finally, the systems can automatically generate a medication administration record (MAR) and seamlessly feed this information into the appropriate hospital information system.
Common Data Standards,
Well-designed Interfaces
The IOM recommends that all medication-safety technologies be electronically linked to enable an integrated view of demographic, clinical, and medication history, vital signs, laboratory results, intake/output, and patient response to medication. However, the report also states that realization of the full benefits of many healthcare information technologies (ITs) (such as decision-support systems, smart IV pumps, barcode administration systems and pharmacy database systems) is hampered by the lack of common data standards for system integration and well-designed interfaces for end users (IOM, 2006).




Efforts such as Health Level 7 (HL/7) and the Medical Information Bus (IEEE 1073) to integrate various elements are to be applauded. However, until now, no one organization has had all of the assets, technologies, IT systems, and interfaces needed to integrate systems for all elements of the medication-use process, i.e., inventory management, drug distribution, dispensing, administration for both non-infusion medications and IV infusions, documentation, surveillance, alarms and alerts management, data analysis, and billing. If a hospital has many different products from multiple vendors, integration becomes extremely challenging and can compromise safety, productivity, and data quality.
It is critically important that the data flow in a way that is user-friendly and not compromised by the presence of differing structures and architectures with conflicting rules. As yet no vendor has achieved complete integration between all pieces; however, hospitals should look to partner with a vendor with the highest capabilities to achieve this objective. When an organization has all the necessary assets with one set of rules, analytics, and reports, then the vision of a fully integrated, safer and simpler medication-use system can become a practical reality (Figure 3).
Conclusions
Through its series of reports, the IOM has greatly increased awareness and spurred efforts to improve medication safety. The complexity of the medication-use process is recognized as a major contributor to medication error. Reducing complexity by simplifying, standardizing, automating, and integrating the various elements of this process is essential.
An organization needs to effectively manage the entire medication-use process. Building safety into the system requires safeguards at every step from procurement to the point of care, not just at the beginning or end of this highly complex process. It is important to look at the medication-use process in its entirety, take out non-value-added steps, and build in intelligent redundancies to improve efficiency, operational performance, and safety for the entire system. Redundant checks are the foundation of high reliability.
There is no "one size fits all" solution. Hospitals vary widely in terms of size, purchasing practices, current technologies, workflow, resources, etc. To find solutions to meet their particular needs, hospitals may benefit from a strategic partner that provides people, products, technologies and processes to improve patient safety, efficiency, financial performance and quality of care. Collaborating with a strategic partner with a shared vision creates the opportunity to develop an integrated, enterprise-wide medication-management system for all medications, benefiting both partners and, most importantly, the patients whom they serve.
Tim Vanderveen is vice president of Cardinal Health's Center for Safety and Clinical Excellence. He is responsible for ensuring the Center's commitment to education and innovation to reduce variation in clinical practice, and to supporting hospitals' patient safety initiatives. From 1972 to 1982, Vanderveen was the director of clinical pharmacy at the Medical University of South Carolina. Since 1983, he has been in the medical device industry, with IMED, Alaris® Products, and most recently Cardinal Health. He has been instrumental in many of the safety and performance enhancements and safety innovations in drug infusion. Vanderveen has authored numerous patents, is a frequent speaker on medication safety at educational programs, and routinely contributes to the medical, pharmacy, and nursing literature. He may be contacted at Tim.Vanderveen@cardinal.com.
Sherri-Lynne Almeida is director of Cardinal Health's Center for Safety and Clinical Excellence. She has 26 years of nursing experience in emergency care and infectious diseases and is a member of the Academy of Emergency Nursing. Almeida is a member of the Emergency Nurses Association (ENA) Board of Directors, having previously served on the ENA Board for 7 years prior to becoming the National President in 2002. Almeida is an assistant professor at the University of Texas Graduate School of Nursing in Houston.
Stephen Lewis is chief medical officer for the Clinical Technologies and Services (CTS) business segment of Cardinal Health. He leads the Clinical Standards and Practice area — the Center for Safety and Clinical Excellence — which provides advice, expertise, and oversight around all clinical practices, and establishes the clinical direction for CTS. Lewis is responsible for establishing clinical protocols, aligning clinical strategies with business strategies, and supporting safety and quality efforts at the bedside and point of care. Previously, he was vice president of medical affairs at VHA Southeast. Lewis is board certified in internal medicine as well as quality assurance and utilization review, and is a member of the American College of Physicians and the American College of Physician Executives.
References
Cardinal Health (2005). Data on file.
Bates, DW. (2001). Personal communication. October.
Hicks, R. W., Cousins, D. D., & Williams, R. L. (2003). Summary of information submitted to MEDMARX in the year 2002. The quest for quality. Rockville, MD: USP Center for the Advancement of Patient Safety.
Hoffman, J.M., Shah, N,D, Vermeulen, L.C. (2006). Projecting future drug expenditures. American Journal of Health-System Pharmacy, 63(2), 123-38.
Institute of Medicine. (2006). Preventing Medication Errors: Quality Chasm Series. Washington, DC: National Academies Press. PDF available at: http://www.nap.edu/catalog/11623.html
Leape, L.L., Bates, D.W., Cullen, D.J., et al. (1995). Systems analysis of adverse drug events. Journal of the American Medical Association, 274, 35-43.
Maddox, R.R. (2005). Use of automation in drug dispensing: the case for decentralized medication cabinets. In: Schneider, P.J. (ed). Drug Dispensing and Administration. HealthLeaders. December (suppl), 3-5.
Maddox, R.R., Williams, C.K., Oglesby, H., et al. (2006). Clinical experience with patient-controlled analgesia using continuous respiratory monitoring and a smart infusion system. American Journal of Health-System Pharmacy. 63, 157-164.
Maviglia, S.M, Yoo, J.Y., Franz, C., Featherstone, E., Churchill, W., Bates, D.W., et al. (2007). Cost-benefit analysis of a hospital pharmacy bar code solution. Archives of Internal Medicine, Apr 23;167(8), 788-94.
Meisel, S. (2006). Safety as a system property. www.swiss-q.org/pdf/meisel.pdf (Accessed Nov 14, 2006)
Pedersen, C.A., Schneider, P.J., Scheckelhoff, D.J. (2006). ASHP national survey of pharmacy practice in hospital settings: dispensing and administration 2005. American Journal of Health-System Pharmacy, 63, 327-45.
Vanderveen, T. (2006). IVs first: A new barcode implementation strategy. Patient Safety and Quality Healthcare, 3(3), 14-20.
Williams, C., Maddox, R.R. (2005) Implementation of an i.v. medication safety system. American Journal of Health-System Pharmacy, 62, 530-6.
|
 |
 |
 |


















|
 |



