Intravenous Infusion Medication Safety: The Vision Becomes Reality
March/April 2013
![]()
Intravenous Infusion Medication Safety:
The Vision Becomes Reality
In 2000 the Institute of Medicine (IOM) published To Err Is Human, the first in its groundbreaking series of reports that brought the issue of medication safety to national awareness. The Institute for Safe Medication Practices (ISMP) February 2002 Medication Safety Alert! suggested that “with computerized prescriber order entry (CPOE), barcode medication administration (BCMA), and now smart infusion pumps, we may finally have a solid defense against the most serious medication errors” (ISMP, 2002). While these three technologies have played a major role in improving medication safety, there are still opportunities for errors.
|
Optimizing the Medication Use Process Invited Conference Faculty |
For example, an infusion pump has no way of knowing if the drug being infused is the correct drug or if the dose is actually the ordered dose. The programming of an intravenous (IV) infusion pump may not match the physician’s order in the CPOE system. Even though an order to discontinue a drug has been entered in the system, infusion may continue without a valid order. High-risk medications such as opioids are frequently administered to patients while relying on only intermittent spot checks of patients’ respiratory status, not advanced respiratory monitoring technology, to guard against potential opioid-related respiratory depression. Without integration of these technologies into a comprehensive medication system, moving to a higher level of medication safety will be difficult.
Wireless connectivity has enabled bi-directional communication, but going from bi-directional communication to interoperability among multiple devices and systems is exponentially more difficult. Today, through ground-breaking collaboration between medical device vendors, health information technology (HIT) vendors, and leading-edge, early-adopter hospitals and healthcare systems, this is happening.
In a 2007 PSQH article, my co-authors and I described a future vision of what the integration of medication safety technologies and HIT could provide (Vanderveen et al., 2007). Today much of what was envisioned in that article is in the beginning stages of being fulfilled. Recognizing the very significant changes currently underway, in June of 2012 the CareFusion Center for Safety and Clinical Excellence hosted an invited conference that brought together early adopters of technology (see sidebar) to help clarify where we are now and consider future possibilities to further optimize the medication-use process.
 |
|
| Figure 1. © 2013 CareFusion |
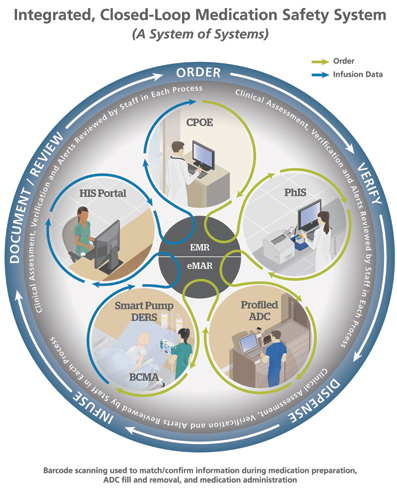
In this article, I review technology adoption and leading-edge innovations related to IV medication use, from initial review of the physician’s orders to ultimate disposal of pharmaceutical waste. It is exciting that integration and interoperability have now become possible as we finally realize the vision of a closed-loop medication safety system that protects all types of medication administration, including highly complex IV infusions (Figure 1).
Technology Adoption and More Advanced Functions
Opening the conference, Phil Schneider reported on the latest American Society of Health-System Pharmacists survey focused on dispensing and administration (Pedersen et al., 2012). Decentralization of the medication-use system continues, with 40% of hospitals using a decentralized system and 58% of hospitals planning to do so in the future. Automated dispensing cabinets (ADCs) were used by 89% of hospitals; robots, by 11%; carousels, 18%; and machine-readable coding was used in 34% of hospitals to verify doses before dispensing. BCMA was used in 50% of hospitals, and 68% of hospitals had smart infusion pumps. Health information is becoming more electronic, with 67% of hospitals having partially or completely implemented an electronic health record (EHR) and 34% of hospitals having CPOE. The use of these technologies has substantially increased over the past year (ASHP, 2012). The following descriptions are examples of advanced medication-safety technology use.
Order Review
Telepharmacy
More than 60% of hospitals now have 24-hour review of medication orders by pharmacists, and 11.1% use a national or regional telepharmacy company to provide such services when the hospital pharmacy is closed (Pedersen et al., 2012). State-of-the-art telecommunications now enable pharmacists to perform pharmacy services at a distance.
Tony Antonopoulos reported on the use of outsourced order entry and review during conversion to an EHR at St. Vincent Hospital in Indianapolis, a large quaternary-care institution. Shortly before conversion, plans were changed to include all areas except the operating room. The additional areas accounted for an estimated 10% of the order-verification workload, and the hospital had to provide for the suddenly increased demand. The contracted outsourced service was up and running in less than 30 days. The service helped minimize labor costs, and nursing was satisfied with the service. The hospital is now reviewing the potential for remote clinical services as part of optimal pharmaceutical care based on facility and setting needs.
Preparation
Automated IV Medication Compounding
Preparing IV medications presents major safety challenges. In particular, after technicians compound a drug in one room, a pharmacist in another room who has not seen what they have done is expected to check a clear liquid in a clear liquid and sign off that everything is correct. In one case, a pharmacist was held responsible for a technician’s fatal error in preparing chemotherapy (ISMP, 2009).
Jack Bond and Caryn Belisle reported on the use of technology to improve IV compounding accuracy, in particular, the use of robots to compound injections and infusions precisely and document the process to aid pharmacy review and regulatory compliance. A web application allows a pharmacist to log onto the system from anywhere in the hospital, remotely view all the information on an IV preparation, see images of the actual vials that were used, see a picture of the final product and sign off remotely. The technology also guides technicians through the compounding process using barcode scanning, controls quantity accuracy to ISO+ standards, and guides the technician to refill if quantity does not fall within standard operating procedures.
Pharmacy Logistics
Ray Maddox reported on experience at St. Joseph’s/Candler Health System (SJCHS) in Savannah, Georgia, with use of a near-real-time software application for pharmacists that provides infusion-status data from pumps and syringe modules, shows safety-software soft-limit violations, and provides easy-to-view information on infusions in progress. This information can be used to help smooth workflow, track infusion alerts, and ensure timely medication delivery from pharmacy to bedside. Maddox noted the potential of pharmacy logistics to improve compounding and infusion efficiencies for pharmacy and nursing, reduce IV waste, and decentralize safety-software oversight to bedside clinicians.
Distribution
Increasing Use of ADCs
ADCs illustrate how medication safety technologies continue to evolve. Early ADCs held a bulk supply of medicines for nurses to obtain as needed with no double check by pharmacy. The development of “profiled” ADCs overcame this problem by linking the pharmacy information system to the ADC. With the exception of overrides, medicines could not be obtained without prior pharmacist review. A seven-hospital study showed that as the percent of medications distributed through the ADC pathway increases, the predictability of medication availability increases, while the total time to initial dose, number of missing doses, and pharmacy labor decrease. (Baker, et al.)
IV Medication Administration
Integrating Technologies—Closed-Loop Medication Safety System The vast majority of larger hospitals have budgeted for or already have the various technologies needed to take the next step: integrating multiple devices and systems to create a closed-loop medication administration safety system (Table 1). Jeff Fleming reported that Children’s Hospitals and Clinics of Minnesota in Minneapolis has become the first pediatric hospital to achieve interoperability between BCMA, smart pumps, and electronic medical records (EMR) (Figure 1).
The vast majority of larger hospitals have budgeted for or already have the various technologies needed to take the next step: integrating multiple devices and systems to create a closed-loop medication administration safety system (Table 1). Jeff Fleming reported that Children’s Hospitals and Clinics of Minnesota in Minneapolis has become the first pediatric hospital to achieve interoperability between BCMA, smart pumps, and electronic medical records (EMR) (Figure 1).
The system safeguards large-volume and syringe-pump infusions in critical and non-critical care areas. BCMA technology has been available for over a decade; however, it was not capable of confirming that an infusion pump was programmed correctly. In addition, unlike other medications administered with BCMA, the infusions were not automatically captured in the EMR. Automatically sending infusion orders directly to each smart pump and infusion data back to the EMR helps ensure correct infusion programming and timely, accurate capture of infusion data, which the nurse can then approve and enter (Table 2). This full, closed-loop system secures the 6th Right of medication administration—right documentation.
The EMR system also links with information systems that report laboratory values, note discontinued medications, allergies, and provide clinical decision support. In critical care areas, infusion management (IM) technology displays infusion and other patient data such as vital signs and hemodynamics in real time. Clinicians can now monitor the status of infusions and related data from any location across the hospital. Server-based data-management applications can track and manage hundreds or even thousands of infusion devices enterprise-wide.
|
Table 2. Closed-Loop Medication Administration
Ideal workflow: Scan the patient, verify correct patient, scan medication, verify correct medication, time, dose, route; document medication; scan channel; verify correct programming of pump; save to the EMR; and review results in the infusion management (IM) dashboards along with other patient data (vital signs, hemodynamics) in a real-time visual graphic display |
PCA and Respiratory Monitoring
Integrating respiratory monitoring with PCA and epidural infusions helps ensure the 7th Right – right response of the patient. Oversedation can lead to fatal opioid-related respiratory depression. Individual response to opioids varies greatly, and it is not possible to prospectively identify all patients at increased risk (Maddox et al, 2006).
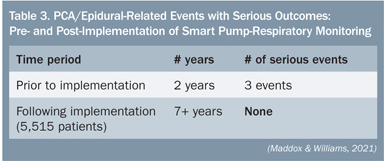 SJCHS was the first hospital system to implement an IV medication safety system that combines continuous capnography with pulse oximetry modules and “smart” PCA infusion pumps on a single platform. The system provides information on the patient’s physiological response to PCA therapy and gives clinicians an early warning of potential respiratory depression. When the PCA and monitoring modules are used as part of the overall system, if a patient falls below hospital-defined respiratory limits, the system’s unique “pause protocol” automatically pauses the PCA infusion and deactivates the dose-request cord (Maddox et al, 2008). As show in Table 3, in the more than seven years since the implementation of PCA-respiratory monitoring, there have been no serious PCA- or epidural-opioid-related events at SJCHS (Maddox & Williams, 2012).
SJCHS was the first hospital system to implement an IV medication safety system that combines continuous capnography with pulse oximetry modules and “smart” PCA infusion pumps on a single platform. The system provides information on the patient’s physiological response to PCA therapy and gives clinicians an early warning of potential respiratory depression. When the PCA and monitoring modules are used as part of the overall system, if a patient falls below hospital-defined respiratory limits, the system’s unique “pause protocol” automatically pauses the PCA infusion and deactivates the dose-request cord (Maddox et al, 2008). As show in Table 3, in the more than seven years since the implementation of PCA-respiratory monitoring, there have been no serious PCA- or epidural-opioid-related events at SJCHS (Maddox & Williams, 2012).
The ISMP and the Anesthesia Patient Safety Foundation have recommended adoption of continuous monitoring of patients receiving opioid infusions (ISMP, 2003; Weinger, 2006-2007). Implementation of “smart” PCA pumps combined with continuous respiratory monitoring also can help hospitals comply with Joint Commission standards for effective pain management, while improving medication safety and quality of care (Maddox, 2008).
Disposal
Managing Pharmaceutical Waste
Fred Massoomi reported on Environmental Protection Agency (EPA) requirements that pharmaceutical waste be managed safely from the moment it is generated to when it is appropriately disposed. Pharmacists may be held personally liable for lack of compliance (Deegan, 2005).
An automated pharmaceutical waste management system uses barcode technology to identify drugs and a database of more than 180,000 National Drug Code products to categorize and separate pharmaceutical waste at the point of disposal. The system also documents and tracks pharmaceutical waste and generates customer and administrative reports. Automating the identification and sorting of residual drugs eliminates the human error and potential cross contamination inherent in manual sorting of pharmaceutical waste. Accurate and efficient sorting of waste is also financially prudent. Indiscriminately disposing of pharmaceutical waste by throwing it in the trash or into the sewer system is no longer acceptable (ASHP Discussion Guide).
Issues/Concerns
Continuing Challenges
Maddox noted that while we have seen tremendous technological achievements, gaps still exist that need to be filled. For example, medication-safety technology can only capture near-miss errors specific to the technology, e.g., smart pumps only capture infusion data. Errors not prevented by BCMA include a physician’s order in the wrong patient chart, wrong time relative to meals, IV medications not given via pump, medications not crushed or chewed, failure to check vitals around medication admin, failure to filter IV medication, etc. Nursing education on proper administration of medications is the key to decreasing such technique errors.
HIT Safety Concerns
Phil Schneider was a member of the IOM panel that recently published Health IT and Patient Safety: Building Safer Systems for Better Care (IOM, 2011), which notes that current literature is inconclusive regarding the overall impact of HIT on patient safety. While HIT has demonstrated potential to improve patient safety, it also can cause harm. Schneider emphasized that all stakeholders, including the private and public sectors, must coordinate efforts to increase our understanding of risks associated with health IT and improve its safe design, implementation, and use. Pharmacists can, should, and will have an important role in the acquisition, implementation, use, and refinement of health IT systems
Freeing Pharmacists/Nurses for More Clinical Activities
A major goal of all these technology innovations is to allow pharmacists and nurses to devote more of their time to clinical activities, as called for by the Pharmacy Practice Model Initiative of the American Society of Health-System Pharmacists (ASHP, PPMI) and the Transforming Care at the Bedside initiative of the Institute for Healthcare Improvement (IHI) (Rutherford, 2008). Optimizing the medication use process can help achieve this goal. Using ADCs to distribute almost all medications can allow a single pharmacist to oversee the drug distribution even at a multi-hospital system. Many of these innovations greatly benefit nursing. For example, while critical titrations are being performed, a closed-loop system automatically captures all infusion data and allows the nurse to concentrate on patient response without having to document changes at the time or subsequently try to recall exactly what was done when.
Conclusion
As discussed in the 2007 PSQH article, to achieve the ultimate goal of a fully integrated infusion safety system, clinical staff, clinical engineering, and IT professionals will need to work together, understand each others’ needs, and recognize that their disciplines will continue to converge. This presents both cultural and technological changes. However, as such changes are embraced, the results are remarkable.
As Carlos M. Nunez, MD, chief medical officer at CareFusion, pointed out at the close of the Center’s conference, “As hospitals and healthcare systems move rapidly into an era where actionable information drives the most fundamental decisions, the interoperability between medical devices and information systems will become increasingly important in improving patient safety, clinical outcomes, staff productivity, and financial performance.”
Tim Vanderveen is vice president of CareFusion’s Center for Safety and Clinical Excellence in San Diego, California, and may be contacted at tim.vanderveen@carefusion.com.
References
