MRI Suites: Safety Outside the Bore
September / October 2006
MRI Suites: Safety Outside the Bore
![]()
MRI suites hold unique dangers for patients and staff. Today’s high-strength clinical MRI scanners are up to 60,000 times the strength of the Earth’s own ambient magnetic field. While exposure to magnetic energies has shown no harmful biological effects — unlike modalities that rely on ionizing radiation such as CT or conventional X-ray — there are still many accidents and incidents that jeopardize the safety of patients and staff in the MRI suite.
The proliferation of MRI equipment and significant increases in both magnet strength and spatial gradients (the rate at which magnetic field strength increases as you approach its center) have increased the number of accidents occurring in the MRI suite. Each accident and close call puts patients and staff at risk and carries the potential of damaging, if not crippling, over a million dollars worth of imaging equipment.
![]()
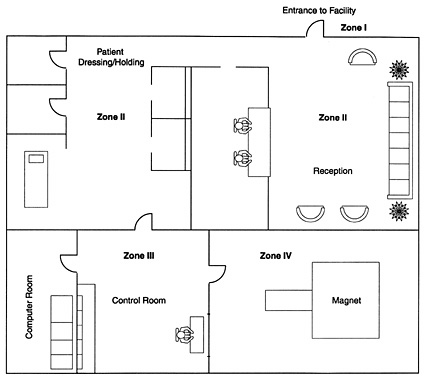
Like a tripod, MRI safety is comprised of three integral components: clinical safety, operational safety, and facility safety. Neglecting any one constituent part jeopardizes the efficacy of MRI safety initiatives. Clinical and operational components of MRI safety have been the subject of significant inquiry and many published papers. Facility safety, by contrast, has long been the “weak link” in an effective program for reducing accidents and facility liability.
Which MRI Risks Can Suite Design Mitigate?
Biostimulation Interference
While magnetism doesn’t pose a biological hazard to people, magnetic fields can disrupt implanted (or on-planted) medical devices. Devices as varied as pacemakers, cochlear implants, implanted insulin pumps, and Vagus nerve stimulators all can be impaired or incapacitated, resulting in significant injury or even death.
In response to this risk, MRI equipment manufacturers and government regulators recognize the 5-gauss exclusion zone. In essence, access to this portion of the three-dimensional bubble of magnetic force is to be limited to only those persons who have successfully cleared screening for contraindicated devices.
Fortunately, many contemporary magnet systems have so effectively compressed the reach of the stray magnetic field through active shielding technologies that, in many suites, the 5-gauss line can be completely enclosed within the magnet room. However, either because of the strength of the magnet or the simple three-dimensionality of the magnetic field, 5-gauss threats can penetrate the magnet room enclosure and present themselves in adjacent areas, including above and below.
This single codified standard, by virtue of its inclusion in MRI vendor siting documents, is the only standard of facility safety incorporated in the planning and design of many MRI facilities.

Missile Effect
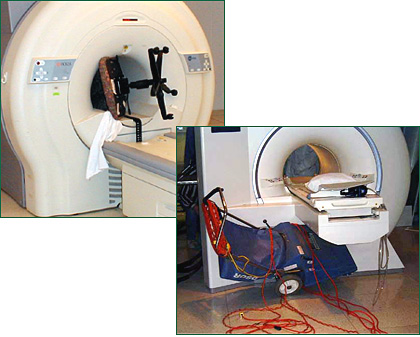
Also referred to as the projectile effect, the missile effect is the tendency of the extreme strength of contemporary MRI magnets to draw ferromagnetic materials into the center of the magnet. Iron-containing materials, including steel, can be drawn to an MRI with such force that they become airborne, accelerating at speeds of up to 40 miles per hour.
The attractive force exerted by MRI magnets on ferromagnetic objects has frequently and repeatedly resulted in accidents jeopardizing the safety of patients and staff, as well as the MRI equipment itself (See figure 1).
In fact, 2006 marks the 5-year anniversary of the most infamous missile effect fatality, that of a 6-year-old post-operative patient receiving a follow-up scan at Westchester Medical Center in New York. This accident occurred when an oxygen cylinder was brought into the MRI scan room while the young boy was inside the bore of the magnet. The magnet grabbed the steel cylinder and drew it into the bore, where it crashed into the boy, killing him.
Cryogens
Most contemporary high-field (1 Tesla or higher) clinical MRI systems rely on liquid helium that is more than 450 degrees Fahrenheit below zero. Approximately 1,000 liters of liquid helium resides in a cryostat, which is essentially a thermos bottle inside the MRI. While helium is non-toxic, the physical properties of cryogenic liquids are very dangerous.
While the risks are greatest when volumes of liquid helium are brought to the magnet to fill the cryostat and replace escaped helium, there is an omnipresent risk of a specific magnet failure, called a quench, when temperature inside the cryostat exceeds the allowable limit and the cryogens boil into a vaporous state, bursting through an escape valve.
The profound thermal expansion of boiling liquid helium is similar to a small explosion, which blasts super-cold vaporous helium from the magnet vessel. Nearly all clinical superconducting magnets have a cryogen vent or quench pipe, which is essentially a chimney intended to duct escaping cryogens to some safe discharge point. From the pressure valve, escaping cryogenic gas is supposed to be ducted to outside the building, frequently an unoccupied rooftop, where it blasts out. However, there have been multiple failures of cryogen vent systems stemming from a variety of sources such as improper design, construction failures, and inadequate maintenance.
If the cryogen vent system fails, hundreds of thousands of liters of gaseous helium can be vented into the magnet room. This super-cold helium, in addition to posing a direct risk of severe cold burns, displaces the oxygen in the room creating an asphyxiation hazard. The expanding cryogenic gas will also pressurize the room. If the door swings into the magnet room, the pressure may prevent opening the door to evacuate any trapped patient or staff member until the pressure is dissipated.
How Significant Are These Risks?
Experts in MRI safety issues believe that these concerns continue to increase, as do both the number and field strength of magnet systems, in the absence of requirements for protection of patient safety.
But where does one turn to quantify the risks? The FDA maintains the only public national database of MRI accidents. This MAUDE (Manufacturer and User Facility Device Experience) database records reported incidents of FDA-approved medical equipment involved in an event that caused “death or serious injury” or presented an “unreasonable risk of substantial harm.” Despite the broad reach of the FDA’s accident reporting criteria, the number of incidents reported is believed to be shockingly low.
Experts familiar with MRI safety issues are often personally aware of many accidents that resulted in serious injury or presented an “unreasonable risk” that have not been reported to the FDA. Dr. Emanuel Kanal, FACR, a prominent neuroradiologist and authority on MRI safety issues, has indicated that the reported incidents catalogued by the FDA are “not even the tip of the iceberg. (MacNeil, 2005)” Estimates are that the MAUDE database accounts for between 1% and 10% of the actual number of accidents, and Dr. John Gosbee, director with the VA’s National Center for Patient Safety, believes the number of incidents in the FDA’s records to be much closer to the low end of the estimated range (personal communication, 2006).
This pervasive failure to report accidents has bred an environment in which MRI providers and both clinical and risk managers profoundly underestimate the risks to patients, staff, and equipment.
Based on anecdotal and published accounts of accidents and close calls, it appears that the number of incidents is increasing. As magnet strength increases with successive generations of MR technology, and as active shielding further condenses the attractive forces of magnetic fields into ultra-concentrated zones, further increases in the number of accidents and incidents are likely.
Those who base their risk-assessment on public accounts or peer-reviewed articles on MRI accidents are likely to be underestimating the potential risks by up to two orders of magnitude. Further developments add to this “risk-awareness deficit:” improving MRI technology increases some risks, and we are using MRI to image and treat a growing diversity of the elderly, infirm, and acute patients. The risks to patients, staff, and equipment are very real today; without intervention to improve current safety provisions, the risks will become increasingly severe in the years ahead.

Who Is Responsible for Suite Safety?
We all recognize that healthcare is heavily regulated. Radiology, in particular, because of the use of ionizing devices, has multiple layers of regulation and oversight. However, because it does not use ionizing radiation, MRI escapes virtually all of the additional regulations imposed on other radiology devices and treatments.
Because of the extreme complexity in siting MRI equipment and the volumes of technical information provided by MRI manufacturers, there is a popular assumption that vendor-provided siting information addresses global MRI safety issues. MRI equipment vendors provide siting information to assure a benchmark level of image quality and device functionality, not facility safety. Vendor-provided floor plans notwithstanding, the safety of suite design and operation are the exclusive responsibility of the facility owner or operator and their agents.
So if the vendor who provides the MRI doesn’t address the safety of the MRI suite, who does?
The United States Food and Drug Administration (FDA), which provides extensive regulations covering manufacturing and marketing of medical devices, has no authority over the physical siting and operational aspects of MRI suites. Individual states, which tightly regulate intentional and incidental exposure to ionizing radiation, generally have no regulations or formal guidance addressing the risks associated with MRI’s high-strength magnetic fields. And while it is the policy of the United States Occupational Safety and Health Administration (OSHA) that the 5-gauss line be restricted around MRI suites, has anyone ever heard of an OSHA citation of an imaging provider for failure to control the 5-gauss line? The Joint Commission on the Accreditation of Healthcare Organizations (JCAHO), charged with protecting patient safety and promoting best practices for member facilities, typically only inquires about fire extinguishers when evaluating the physical safety provisions in the MRI suite.
The physical design standard referenced by JCAHO and many state departments of health is the Guidelines for Design and Construction of Health Care Facilities published by the American Institute of Architects (AIA). The current version (2006) of this document offers only five sentences of guidance for the design of MRI facilities. The American College of Radiology (ACR), which has specific provisions for the accreditation of MRI facilities, does not include patient safety as a part of its accreditation process, despite having published two iterations of the authoritative ACR White Paper on MR Safety.
In short, there is almost no design guidance available, from either regulatory or professional bodies, to facilities looking to protect patients, staff, and millions of dollars of equipment.
What Can Be Done to Mitigate These Risks?
Henry Ford revolutionized industrialized production with the implementation of the assembly line. The McDonald’s Corporation kick-started much of the study of ergonomics with their investigations into optimal workflow and efficiency. What can Model-Ts and cheeseburgers teach us about patient safety? A great deal, as it turns out. Both the Ford Motor Company and McDonald’s realized that the optimization of a work process requires rethinking the space provided to support that process.
Physical process improvements don’t have to be limited to industrial production or mass-market profit. Patient safety in the MRI suite can be significantly enhanced by a facility specifically designed to support these processes.
Patient screening processes are enhanced when private interview areas are provided. More effective clinical screening can reduce the likelihood of missed contraindications. Providing changing areas and the use of contemporary ferromagnetic detection systems specifically designed for the MRI environment can significantly reduce the introduction of ferromagnetic threats. Designs for magnet rooms that include emergency exhaust systems and pressure-relief systems can virtually eliminate potential risks of entrapment from cryogen quench breach. These examples, along with hundreds of other design-related considerations, can improve safety and minimize the risks of accidents in the MRI suite.
If the causes of accidents are easily identified and can be effectively interdicted, why haven’t these been addressed before in codes and standards? Significant progress in MRI safety has been made in clinical venues, but the interdependency of clinical, operational, and facility safety issues often thwarts a more comprehensive approach. Establishing a three-way dialogue with clinicians, departmental administrators, and a facility’s engineering or design staff proves to be a Sisyphean task at many facilities. When this responsibility is delegated to a single discipline, the breadth of perspective is lost, and proposed interventions may fail to incorporate improvements that are apparent to other vantage points.
The safety of patients is a clinical concern, but strategies for improving patient safety don’t always fall exclusively in the clinical domain. Improving the processes of patient care within the MRI department requires that we transcend the divisions on the facility’s organizational chart and marry the experiences of MRI facility design experts with departmental management, radiologists, and technologists in an interdisciplinary team. Facilities that find a lack of in-house expertise in any one of these areas may be well advised to bring in outside consultants to help develop a comprehensive MRI safety program that addresses each of the constituent parts.
Is It Worth the Trouble?
If improved patient safety isn’t perceived as its own reward, there are significant financial considerations at stake in an MRI facility.
If a facility’s MRI scanners are being effectively utilized, each magnet can represent a daily source of $10,000 in revenue or roughly $20 per minute. Accidents that require magnet service or repair can have total costs of hundreds of thousands of dollars. Consider also the financial and marketing costs of the ongoing $10 million lawsuit against Westchester Medical Center arising out of the tragic 2001 missile-effect fatality. But at MRI’s rates of revenue, it doesn’t require a catastrophic event to see negative effects. Even accumulated minor interruptions can have significant financial impacts to a facility’s financial performance.
Improvements in an MRI facility’s process and safety resulting from improved suite design will pay dividends in the form of reduced scanner downtime and improved patient throughput. At $10,000 per day in revenue, a 1% improvement in facility efficiency from enhanced design will yield more than $30,000 in additional annual revenue.
MRI suite safety design is not a “silver bullet” that can erase the risks associated with cryogens, magnetic field interactions, or missile — effect incidents. However, when used in concert with clinical and operational measures, facility design can significantly enhance patient safety and operations. Particularly given the risk factors that are on the increase — increasing patient acuities, increasing numbers of interventional and sedation exams, stronger magnetic fields, and greater attractive forces — failure to implement the best practice methodologies of MRI facility design will increase the risks of injury to patients and staff and the corresponding liability for the MRI facility.
References
McNeil, D. (2005, August 19). M.R.I.’s strong magnets cited in accidents. New York Times.
Web Resources
ACR White Paper on MR Safety (2004 Edition). www.acr.org/s_acr/ bin.asp?TrackID=&SID=1&DID=12183&CID=1848&VID=2&DOC=File.PDF
AIA. (2005, October 19). New standard of practice for the design of MRI facilities. The Academy Journal. www.aia.org/journal_aah. cfm?pagename=aah_jrnl_20051019_mri&dspl=1&article=article
AIA Guidelines for Design and Construction of Healthcare Facilities (publication information). www.aia.org/aah_gd_hospcons
FDA’s Center For Devices and Radiological Health. www.fda.gov/cdrh
FDA’s Medical Device Reporting Requirements. www.fda.gov/cdrh/ devadvice/351.html
VA National Center for Patient Safety MR Hazard Summary. www.va.gov/ncps/SafetyTopics/mrihazardsummary.html

