Look-Alike Drug Name Errors
July / August 2010
Look-Alike Drug Name Errors
Is Enhanced Lettering the Answer?
Medication errors can result in significant morbidity and mortality and more costly care. Look-alike drug names that contribute to wrong drug errors are pervasive. These errors happen in various ways; for example:
Prophylactic antibiotic coverage with penicillin 500mg oral tablets four times a day was ordered for a 43-year woman in intensive care with severe mitral and tricuspid valve regurgitation and an incidental jaw fracture who was scheduled for a surgical valve repair. On the second day of antibiotic therapy, a nurse compared the drug she was to administer with the medication administration record and discovered the patient was receiving penicillamine instead of penicillin. Further evaluation revealed the staff member who entered the order into the pharmacy computer system typed in “PENIC” and had received a drop-down box that displayed all the formulations starting with “PENIC.” The pharmacist entering the order unintentionally selected penicillamine instead of penicillin (Flynn, 2006).
A 40-year-old woman with bipolar disorder (on trazodone) received handwritten prescriptions for Zyrtec® and Atarax® to treat a new episode of urticaria. The pharmacist dispensed Zyprexa® instead of Zyrtec. It was not until a 2-week follow-up appointment that the error was identified (Cohen, 2003).
Epinephrine and ephedrine were available on override in adjacent sections of an automated dispensing cabinet drawer. When a physician requested a dose of ephedrine for a patient in labor, a nurse hurriedly drew up a dose of epinephrine and handed it to the primary nurse, who administered the dose. The patient experienced chest pain and a period of hypertension before the baby was born, however, there were no long term effects for the mother or newborn (ISMP Medication Error Reporting System, n.d.).
These errors have one common characteristic—drug name pairs that look-alike: Zyprexa/Zyrtec, ephedrine/epinephrine, and penicillin/penicillamine. In this article, we investigate why look-alike drug errors occur, how to prevent them, and if enhanced lettering is a solution to this problem.
Look-Alike Drug Name Confusion
The potential for confusion and medication errors associated with the use of look-alike drug names has a long-standing history. Understanding the extent to which look-alike drug names result in medication errors has been difficult because of inadequate and under-reporting of such errors.
In 1998, the U.S. Pharmacopeia (USP), introduced MEDMARX™, a web-based, fully automated national, anonymous, medication error reporting system, which has resulted in an extensive database of voluntary reports. From 2004 to 2008, the proportion of unauthorized wrong drug medication errors attributed to look-alike drug name confusion reported to MEDMARX™ ranged from 11.7% to 14.4% (U.S. Pharmacopeia, 2009).
Some caveats are appropriate for this data. First, seldom is there only one reason for a medication error. Thus, for the errors cited, there were other contributing causes besides the look-alike drug name problem reported. Second, similar drug names may have played a role in additional medication errors cited in the MEDMARX database where the reporting facility did not recognize or select this as a cause of the error.
The 8th Annual MEDMARX report issued in February 2008 by USP detailed problems with 1,470 drug names (over 3,170 name pairs)(Hicks et al., 2008). The report documents harm with 1.4% of events categorized as either look-alike or sound-alike drug name errors, including seven errors that may have caused or contributed to patient deaths. The most common reasons cited in this report for look-alike drug name errors were poor handwriting and not actively reading labels.
In 2004, the Pennsylvania Patient Safety Reporting System (PA-PSRS) classified 11% of the medication error reports as wrong drug errors (Penn. Patient Safety Reporting System, 2004). In these cases, a drug was prescribed, dispensed, or administered in place of another drug. Of those reports, 34% were because of confusion between similar medication names.
In a review of medication errors classified as wrong drug errors, PA-PSRS data revealed that most common name pairs involved in wrong drug errors are those whose names are similar. For example:
- morphine and hydromorphone,
- hydrocodone with acetaminophen and oxycodone with acetaminophen,
- alprazolam and lorazepam,
- MS Contin and Oxycontin,
- and Novolog Mix 70/30 and Novolin 70/30 (Penn. Patient Safety Reporting System, 2007).
The problem of medication errors because of look-alike names led The Joint Commission to turn its attention to the confusion between drug names and the potential harm that can occur. National Patient Safety Goal 03.03.01 (now incorporated in Standard MM.01.02.01) stated that organizations should “identify, review (annually) a list of look-alike/sound-alike drugs used in the organization, and take action to prevent errors involving the interchange of these drugs” (The Joint Commission, n.d.).
Besides the proximate cause of look-alike drug name errors, there are several contributing factors that can play a role in causing these errors. These reasons include: poor copies of drug orders and failure of pharmacy or nursing personnel to clarify unclear orders directly with the prescriber.
To be sure, prescribers are responsible for writing orders that are accurate, complete, and legible. “Illegible” prescriptions result in a delay, while the marginally legible script leaves the reader confident they have deciphered it, although perhaps incorrectly.
Pharmacists who check the final drug product at the point of dispensing against a computer entry, rather than against the original prescription or drug order, lose that final, critical double-check in the medication-use system. This significantly increases the risk of dispensing the wrong medication and causing the patient harm.
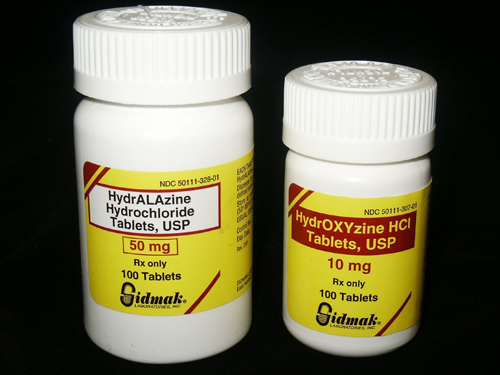
It is far too easy to select the wrong drug from the drop-down list in a computer menu. Using the first three or four letters of a drug name in a pharmacy computer system or computerized prescriber order entry (CPOE) program to look up the medication can be problematic—many drug names begin with the same three or four letters. For example: chlorpromazine and chlorpropamide, tramadol and trazadone, hydralazine and hydroxyzine.
There are many similarly spelled drug names that may be difficult to differentiate on a handwritten prescription order, when selecting a typed name from a menu, or when removing a needed medication from a storage area. ePrescribing has the potential to eliminate much of the confusion because of handwritten orders of look-alike drug names, but it will not end these wrong-drug errors. Relying on healthcare providers to read labels, while important, will not be enough to prevent these types of errors. There are various causes contributing to the problem: being too busy, distractions, interruptions, confirmation bias (i.e. seeing what the reader expects to see), and look-alike drug containers and labeling.
Potential Solutions
Traditionally, education is the strategy of choice to prevent look-alike drug name errors. How often do we hear, “Read the label three times before you dispense or administer the drug to a patient”? Although this admonition is valid, it may not be the most effective strategy in preventing errors associated with similar drug names.
Healthcare professions have expressed patient safety concerns to the pharmaceutical manufacturers to make them aware of the problem of similar brand names for medications. Manufacturers have also been strongly encouraged to evaluate potential brand names before they submit a new drug application to the Food and Drug Administration (FDA).
There are numerous strategies that national healthcare organizations and practitioners can adopt to address the issue of preventing and reducing look-alike drug name errors. Among these are ePrescribing, barcode scanning technologies, well thought-out (segregated) drug storage, and enhanced lettering. One enhanced form of lettering, known as Tall Man Lettering (TML), is the focus of this article.
Tall Man Lettering
Tall Man Lettering (TML) is the practice of writing part of a drug’s name in upper-case letters to help distinguish look-alike drugs from one another to avoid medication errors (Figure 1). For example, in TML, “prednisone” and “prednisolone” would be written “predniSONE” and “prednisoLONE” respectively (Tall Man letering, 2010). The Institute for Safe Medication Practices (ISMP) started using the term Tall Man Lettering (TML) to distinguish similar looking and sounding drug names in 1999 (Institute for Safe Medication Practices, 1999). Wyeth-Ayerst (now Pfizer) was the first pharmaceutical manufacturer to use enhanced lettering with its Tubex® line of prefilled syringe cartridges. After mix-ups between Tubex® cartridges of dimenhydrinate and diphenhydramine, Wyeth began labelling them diMENhydrinate and diPHENhydramine. As a result, fewer mix-ups were reported to the company after this change was implemented (Cohen, 1999).
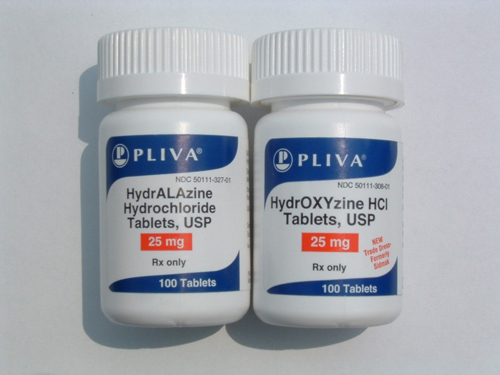
What Is the Evidence that TML Works?
Some studies demonstrate the use of enhanced lettering (of which TML is considered a subset) has improved the readability of product labeling and can reduce the number of errors associated with those products. Grasha (2000) showed that TML reduced drug selection errors by 35% in a simulated pharmacy-dispensing environment.
A second study by Grasha (2003) used enhanced lettering on product labels and NDC numbers in a community pharmacy setting. Labels were designed and placed on a sleeve that fit on the front of stock bottles of 30 products targeted for special attention. The drug names were exaggerated by using color fonts and 18- to 36-point type, which helped people focus on the details of the product during product selection as well as final verification. There was a 27.8% reduction overall in errors when the product sleeves and labels were in place.
Based on a series of controlled laboratory experiments, Filik et al. (2006), found support for the use of TML to reduce errors caused by drug name confusion if participants (n=20) were aware that this was the purpose of the TML. In a fourth study, Gabriele (2006) showed that nurses (n=11) recognized more names with the use of upper-case characters than with boldface characters during word recognition tests.
Research with TML is encouraging, but inconclusive. The use of TML to reduce name recognition errors makes intuitive sense. However, the studies so far with TML have used small numbers of participants under laboratory conditions, which makes external validity suspect. Additional controlled studies, powered correctly, using practitioners in real-world environments will be needed to measure the effect of TML on potential look-alike errors. Without more conclusive evidence from TML studies, the authors suggest that this unique labeling process should continue to be used.
Which Drug Pairs Should Have Tall Man Lettering?
Lambert et al. studied problems with look-alike and sound-alike drug names. In a 2001 study, they concluded that both orthographic (i.e. spelling) and phonological (i.e. sound) similarity increased the probability that experts and novices will make false-recognition errors when trying to remember drug names. What was most interesting about this study was the use of a tool (bigram similarity with Dice coefficient) to measure the extent (low = 0 to high = 1) of similarity between drug pairs. For example, the bigram orthographic similarity score for Prolixin® and Procolin® was 0.78, while the bigram orthographic similarity score for Trantoin® and Triacin® was 0.47.
In a second study, Lambert et al. (2002) tested how well the trigram similarity measure predicted lay participants’ subjective judgments of similarity between drug names. The investigators concluded the Dice/trigram measure of orthographic similarity between drug names is a “valid, albeit incomplete, measure of lay persons’ subjective judgments of the similarity between drug names.” The authors go on to say “… real world drug-name confusions can be minimized with the use of objective measures of similarity during the preapproval screening process for new drug names.”
With the goal of preventing medication errors that have been documented with certain look-alike generic names, the FDA Office of Generic Drugs conducted the Name Differentiation Project in 2001. They communicated recommendations to pharmaceutical manufacturers to change the appearances of these names by changing select lower-case characters in each name to upper-case characters, referred to as “Tall Man Letters.” One hundred and forty-four (144) manufacturers of the 16 look-alike generic drug name pairs were requested to use TML when expressing drug names on labels and packages. So far, compliance with this request is unknown.
Lists of drug name pairs the FDA and ISMP feel deserve consideration for TML are available (U.S. Food & Drug Admnistration, n.d.; Institute for Safe Medication Practices, 2008). However, it is important the number of drug pairs with TML be restricted to look-alike drug name pairs that have a high bigram similarity, or eventually TML may stop catching people’s eyes (Lesar, 2008).
Practitioner Awareness of TML
Both ISMP and USP have recently surveyed practitioners concerning TML. In 2007, USP surveyed organizations of pharmacists, pharmacy technicians, doctors, nurses, and nurse practitioners. Highlights of the responses from 1,788 participants to the USP survey include:
- Awareness of TML among those actively involved in patient medication activities was high at nearly 80%.
- Although the use of TML was widespread, how and when it was used varies widely by organization and profession.
- Most respondents saw TML as helpful and effective in preventing errors; however, satisfaction with TML was moderate and respondents saw room for improvement.
- More than 8 in 10 agreed that TML should be used by manufacturers to differentiate between similar drug name pairs, incorporated into drug information databases, and appear on printed materials with medication names.
- Three-fourths (76%) believed a nationally standardized system of TML should be developed, suggesting that many healthcare professionals would find such a resource appealing and useful.
- Overall, TML was understood, valued, perceived to be helpful and effective; however, satisfaction with organizational use was moderate.
- Almost 9 in 10 believed manufacturers should incorporate TML into computer programs and drug information databases and use TML on printed materials.
- Over two-thirds believed a nationally standardized TML system should be developed.
- Few believed TML effectiveness was diluted by overuse.
In 2008, ISMP conducted a survey about TML and received 451 responses. Nearly all (87%) of the responders believed the use of TML by the medical products industry helped to reduce drug selection errors.19 Further, two-thirds of responders (64%) believed that TML has prevented them from dispensing or administering the wrong medication.
Next Steps
Besides studies that validate the merits of TML, there is the need to discover: 1) which drug pairs are best suited for TML, 2) how TML should be structured, 3) if TML should be standardized, and 4) if so, by whom?
Which drug pairs are best suited for TML?
There are steps that can be undertaken to discover which drug pairs need TML. The first one is to mine data from medication error reporting databases, such as MEDMARX, and the FDA’s MEDWATCH national medication error database to determine which pairs of drug names are contributing to most “wrong drug” medication errors. The combination of look-alike names and shared dosage strength(s) may further increase the likelihood of an error and thus the need for action.
Another step would be to apply Lambert’s objective bigram assessment tool for each drug name pair under consideration, and discover a cut-off point, above which a drug name pair would be a candidate for TML consideration. This could be a joint effort between the FDA and USP, with the FDA making the final determination of which drug name pairs need TML.
How should TML be structured?
Clues on how the TML should look were revealed in the study by Gabriele and the survey by ISMP. In the ISMP survey, participants strongly favored upper-case letters to distinguish differences in drug names. In the Gabriele study, participants echoed this sentiment, but also felt that white letters on a black rectangle were the most helpful in differentiating names. However, there is little evidence regarding which of the dissimilar letters in each drug name should be highlighted.
Who should standardize TML?
USP has been studying TML since 2004. Since it sets the standards for drugs in the United States, it seems the most logical organization to develop standards for how look-alike drug name pairs should be labeled. This effort, of course, should be accomplished in collaboration with the FDA, NABP, ISMP, practitioner professional organizations, and the National Coordinating Council for Medication Error Reporting and Prevention (NCCMERP). It is important to recognize the USP sets standards for the drug monographs (generic names) and could serve in an advisory role when proprietary (brand) names are involved. The FDA currently does not have regulatory authority over proprietary names for non-prescription products.
Summary
Look-alike drug name errors continue with limited abatement. Therefore, it can be concluded that: 1) current strategies to avoid these errors should be more fully implemented, and 2), there continues to be a need for new “out of the box” strategies. Although ePrescribing and CPOE systems may be powerful tools to help avert errors involving handwritten orders with look-alike drug names, it will be some time before these systems are more widely implemented across all healthcare settings. Selection errors among look-alike drug names can also occur when the name is printed from a computer program or displayed electronically.
Preliminary data suggest the use of TML may help in reducing look-alike drug errors. It is a logical and cost-effective solution (compared with slowly developing high technology strategies) to the problem of look-alike drug name errors, although patient safety should not be a cost consideration.
Because look-alike drug name errors continue and are causing harm to patients, the authors suggest the U.S. healthcare system cannot wait for further studies that document the merits of using the TML strategy. USP in collaboration with other national healthcare quality and safety organizations should move forward with developing a standardized approach to TML. Once these standards have been agreed upon, the FDA should consider requiring the use of TML for those drug name pairs that have been documented as associated with most look-alike drug errors.
William Kelly is president of William N. Kelly Consulting, Inc. and vice president of scientific affairs for Visante, Inc. He may be contacted at wnkelly@earthlink.net.
Matthew Grissinger is director of error reporting programs at the Institute for Safe Medication Practices. He may be contacted at mgrissinger@ismp.org.
Marjorie Shaw Phillips is medication safety coordinator and clinical research pharmacist at MCG Health in Augusta, Georgia, and clinical professor of pharmacy practice (WOS) at the University of Georgia College of Pharmacy. She may be contacted at mphillip@mcg.edu.
Acknowledgments
Members of the USP Safe Medication Use Expert Committee (SMU EC) from 2005 to 2010 contributed as reviewers of this article. The authors acknowledge and thank them for their participation. Special thanks to former USP staff member Colleen Brennan, R.Ph., for her editorial role and as the USP liaison to SMU EC and to Shawn Becker, RN, MSN, and Mike Heath, RPh, MBA, for their editorial assistance in reviewing this manuscript.
