Learning From Influenza Vaccine Errors to Prepare for COVID-19 Vaccination Campaigns
By The Institute for Safe Medication Practices
© 2020 Institute for Safe Medication Practices (ISMP). This material has been reposted with permission from ISMP. Other reproduction is prohibited without written approval from ISMP.
Consumers have been responding to the advice of healthcare experts and getting influenza (flu) vaccinations in record numbers this year (Blank, 2020), which will help reduce the burden on the healthcare system due to the dual threat of the flu and coronavirus disease 2019 (COVID-19). While this is wonderful news, ISMP has also seen a corresponding increase in the frequency of reported flu vaccine–related errors. Since September 2020, ISMP has received more than 60 error reports associated with the 2020–2021 flu vaccine.
Analysis of flu vaccine–related errors and other harmful or deadly vaccine errors from the past leads to concerns about the monumental COVID-19 vaccination campaigns that may start as early as this month and will run well into 2021 and beyond. It is evident that many underlying causes of flu vaccine–related errors could just as easily lead to errors associated with the new COVID-19 vaccines and the hundreds of millions of doses that will be given (billions globally). This means that it will be crucial for any healthcare provider who plans to stock and/or administer COVID-19 vaccines to learn from these prior vaccine-related errors, anticipate that similar errors could happen with the COVID-19 vaccines, and take the necessary steps to prepare their facilities and healthcare teams in order to mitigate the risk of vaccine-related errors. We hope that providing a description of the anticipated COVID-19 vaccines, along with the causal factors associated with the recent bout of flu vaccine–related errors and other previously reported harmful or fatal vaccine errors, will help healthcare providers anticipate the risks and prepare for one of the largest vaccination efforts in US history with the upcoming COVID-19 vaccination campaigns (Paris & Hopkins, 2020).
Anticipated COVID-19 Vaccines
It is anticipated that two mRNA (messenger ribonucleic acid) COVID-19 vaccines from Pfizer-BioNTech and Moderna, which are both in Phase 3 clinical trials, may receive Emergency Use Authorization (EUA) from the US Food and Drug Administration (FDA) as early as the end of this month (American Pharmacists Association, 2020). Current resources suggest the Pfizer-BioNTech vaccine (30 mcg/0.3 mL after dilution, multiple-dose vial) requires two doses to be administered 21 days apart, and the Moderna vaccine (100 mcg/0.5 mL, multiple-dose vial) requires two doses to be administered 28 days apart. The vaccine storage temperatures are freezing (Moderna) or subzero (Pfizer-BioNTech); however, temporary storage under refrigeration is allowed for a limited time (five days for the Pfizer-BioNTech vaccine, 30 days for the Moderna vaccine) (Moderna, 2020). The Pfizer-BioNTech vaccine can be brought to room temperature and must be diluted prior to use and administered within six hours of dilution. The Moderna vaccine must be used within 12 hours after storage at room temperature or within six hours after the vial has been entered. The Pfizer-BioNTech (www.ismp.org/ext/588) and Moderna (www.ismp.org/ext/589) vaccine labels are displayed on DailyMed and in Figure 1 (labels might change). All of the current COVID-19 vaccines in development will be administered intramuscularly (IM). Other COVID-19 vaccines will likely receive EUA approval in 2021. Some of these vaccines may need a diluent or an adjuvant provided in a separate vial that requires mixing.
Figure 1. Current Pfizer-BioNTech (top) and Moderna (bottom) COVID-19 vaccine vial and carton labels, which could change.

Causative Factors With Errors
Many of the underlying causative factors associated with the recent 2020–2021 flu vaccine errors and certain harmful or fatal vaccine errors in the past could also be factors that lead to errors with the new COVID-19 vaccines.
Look-alike names, labels, packaging. Similar container labels, packaging, and/or vaccine names have contributed to numerous 2020–2021 flu vaccine errors. Name confusion with the flu vaccine is common given that most of the brand names begin with “FLU” (www.ismp.org/ext/581). An example of errors reported due to name confusion involves administering FLUZONE QUADRIVALENT instead of the intended FLUZONE HIGH-DOSE QUADRIVALENT to patients older than 65 years, or vice versa. Given that the COVID-19 vaccines authorized by an EUA will not be using proprietary brand names, there may be confusion related to similarities in the product name “COVID-19 vaccine.”
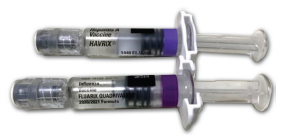
ISMP has also received reported flu vaccine–related errors related to look-alike labeling and/or packaging. For example, practitioners recently reported that prefilled syringes of the flu vaccine FLUARIX QUADRIVALENT and the hepatitis A vaccine HAVRIX, both from GlaxoSmithKline, look very similar (Howard, 2020). Both syringes have a purple band in the same position on the barrel and are similar in shape (Figure 2).
Figure 2. Havrix (top) and Fluarix Quadrivalent (bottom) prefilled syringes look similar in color and shape, and both are refrigerated, contributing to mix-ups.
We also received reports that the white and blue cartons of Fluzone Quadrivalent and FLUBLOK QUADRIVALENT prefilled syringes, both manufactured by Sanofi Pasteur, look very similar with the product names appearing with a similar blue and white color scheme. In one case, a carton of Flublok Quadrivalent was used to restock Fluzone Quadrivalent in an automated dispensing cabinet (ADC), leading to administration of the wrong flu vaccine.
Another report indicated that, for two consecutive years, BOOSTRIX (diphtheria and tetanus toxoids, acellular pertussis [Tdap] vaccine) has been accidentally administered instead of the intended Fluarix Quadrivalent vaccine. The error occurred twice last year and three times this year at different clinics. Both vaccines, from GlaxoSmithKline, come in a white box with a green stylized arch along the left border of the front panel.
Similar mix-ups are possible with the new COVID-19 vaccines if other vaccines or medications have look-alike labels or are packaged in similar-sized and/or colored vials.
Unsegregated storage in a refrigerator. Storing vaccines with other unsegregated vaccines or medications in a refrigerator or freezer has led to mix-ups with serious outcomes. For example, vials of insulin have sometimes been mistaken as flu vaccines, and neuromuscular blocking agents have been used to reconstitute vaccines or were mistaken as the flu vaccine itself. While only temporary storage in a refrigerator is permitted with the new COVID-19 vaccines, if other medications or vaccines are also stored unsegregated in the same refrigerator, mix-ups should be anticipated. Usually look-alike packaging and/or labeling is a contributing factor, but not always. We would be remiss not to mention a few examples of particularly harmful or fatal vaccine errors that have happened during the last six years due to comingled, unsegregated refrigerator storage of vaccines and medications:
- 2019: Four newborns died in Iraq after insulin was administered instead of the intended hepatitis B vaccine (reported to ISMP in October 2020)
- 2019: Two workers and eight residents in a community health facility in Oklahoma were hospitalized, several unresponsive, after insulin was administered instead of the intended flu vaccine (ismp.org/ext/577)
- 2016: Fifty hospital employees in Brazil required hospitalization after insulin was administered instead of the intended flu vaccine (ismp.org/node/549)
- 2014: Five teachers in Missouri, two of whom required hospitalization, received insulin instead of the intended flu vaccine (ismp.org/ext/579)
- 2014: Several patients in a US hospital emergency department received pancuronium instead of the intended flu vaccine, leading to dyspnea and respiratory depression (ismp.org/node/21294)
- 2014: Fifteen children in Syria died after receiving measles vaccine incorrectly reconstituted with atracurium instead of the provided diluent (sterile water) (ismp.org/node/21294)
Numerous 2020–2021 flu vaccine–related errors were caused by selecting the wrong product from a refrigerator. In one case, a nurse reached into the refrigerator and blindly removed a prefilled syringe from a carton of what she thought was Fluzone Quadrivalent but was instead PREVNAR 13 (pneumococcal 13-valent conjugate vaccine), which she did not know was in the refrigerator. She administered the pneumococcal vaccine to a 5-year-old child instead of the flu vaccine. In several other cases during flu vaccination clinics, practitioners mistakenly removed the wrong age-specific flu vaccine from a cooler and administered it.
Mixing errors or omissions with two-component vaccines. Flu vaccines are supplied as a single component, typically as a single dose in a prefilled syringe. However, the high incidence of error reports received by ISMP and others involving two-component vaccines (e.g., vaccine and diluent, vaccine and adjuvant, vaccine liquid and powder components) (Samad et al., 2020) warrants mentioning given that the Pfizer-BioNTech COVID-19 vaccine requires a diluent (not supplied), and subsequent COVID-19 vaccines granted EUA or approval in 2021 may include two components (e.g., vaccine plus diluent or adjuvant liquid) in separate vials. The individual components of two-component vaccines must be mixed together properly, a step that introduces an opportunity for errors. Errors have occurred when only the diluent, adjuvant liquid, or undiluted vaccine itself were administered. Unique storage requirements exist for some of the two-component vaccines, including different temperature ranges for each component. If the two components of a COVID-19 vaccine must be stored separately, or if the labeling on both components looks similar, the risk of an error is heightened. Due to the cold chain requirements of the new COVID-19 vaccines, it is possible that supplies and diluents may ship separately from the vaccine (Centers for Disease Control and Prevention, 2020), which may lead to confusion and the risk of using the wrong diluent. Using the wrong diluent (including a neuromuscular blocking agent as noted above) can result in disastrous outcomes.
Miscommunication. Several 2020–2021 flu vaccine–related errors were associated with language or communication barriers, which can also be expected during mass COVID-19 vaccinations. For example, intranasal FLUMIST QUADRIVALENT was administered to a patient older than 49 years (a contraindication) for whom English was not a primary language, even though the age parameters were fully discussed with the patient. Another flu vaccine–related error occurred due to miscommunication when wearing face masks—a similar condition that will exist and must be considered when administering the new COVID-19 vaccines. Initially, the practitioner administering the flu vaccine misread the patient’s handwritten date of birth. Verbal confirmation of the date of birth was provided by the patient wearing a mask but misheard by the practitioner wearing a mask and face shield. The patient received Fluzone High-Dose Quadrivalent despite being younger than 65 years.
Two flu vaccine–related errors occurred due to incorrect directions provided to vaccinators when supplies of certain vaccines were running low or had been exhausted—again, a condition that will likely exist during COVID-19 vaccination campaigns as limited supplies are initially exhausted. For example, in one case, vaccinators were wrongly told that intranasal FluMist Quadrivalent could be administered to any adult in place of the IM flu vaccines when supplies had been exhausted. FluMist Quadrivalent was administered to numerous patients older than 49 years due to the miscommunication.
Not checking/documenting administration in the immunization information system (IIS). Several flu vaccine–related errors led to repeated doses that had already been administered to patients. However, the opposite type of error happens more often with vaccines that require more than one dose at specific intervals—much like the COVID-19 vaccines that will require two doses separated by 21 or 28 days. Either a patient has received a subsequent dose at the wrong interval or failed to receive a subsequent dose as required. Previously, the vaccines most frequently involved in wrong interval errors reported to ISMP included those that target diphtheria, tetanus, and/or pertussis; hepatitis B; hepatitis A; and the human papillomavirus. More than three-quarters of these errors were associated with not checking the patient’s record or IIS (immunization information system; vaccine registry) to confirm the date of the prior vaccine; lack of prior vaccine documentation in the patient’s record or IIS; or confusing or ambiguous entries in the IIS.
For the COVID-19 vaccines, healthcare providers must bring patients back for a second dose, which may prove to be challenging. Because different COVID-19 vaccines will not be interchangeable, a patient’s second dose must be from the same manufacturer as their first dose (Centers for Disease Control and Prevention, 2020). Thus, providers need to accurately document the vaccine patients received for their first dose and check the IIS prior to administration of the second dose to confirm the required time interval and manufacturer. Providers eligible to administer COVID-19 vaccinations will be required to report this information daily in the IIS (Associated Press, 2020).
Lack of usual procedures/technologies during mass immunizations. Three practitioners reported flu vaccine–related errors associated with the inability to employ the usual procedures and technologies that typically reduce the risk of errors during outpatient vaccinations. Manual activities may replace many of the automated safety checks that typically occur when a vaccine is prescribed and administered to an individual patient. In two events, Flublok Quadrivalent was administered to children under the age of 18 (a contraindication). Both reporters noted that they typically used barcode scanning verification or an independent double check prior to vaccine administration but were unable to do so. While the Centers for Disease Control and Prevention (CDC) has worked with FDA and the manufacturers to include a two-dimensional (2D) barcode with a National Drug Code (NDC) on COVID-19 vaccines, scanning to verify the patient, drug, and dose may not be possible without an individual order for each patient (Howard, 2020).
Temperature excursions and expired vaccines. ISMP received numerous reports of repeated flu vaccinations because the original vaccinations were deemed invalid due to refrigerator temperature excursions, which were often due to inadequate refrigeration, faulty thermostat controls, and refrigeration units with inadequate space to allow good air circulation. With strict temperature requirements needed to maintain stability when storing the two anticipated COVID-19 vaccines, temperature excursions—even a single exposure in some instances—could reduce vaccine potency and lead to wasting precious vaccine doses already in short supply.
Additionally, ISMP received three error reports associated with administration of expired flu vaccines from the 2019–2020 flu season. Because expiration dates may be updated based on vaccine stability studies occurring simultaneously with vaccine distribution, the COVID-19 vaccine vials and cartons may not contain a printed expiration date. The 2D barcode on the vaccine label (if possible) and carton (required) will include a placeholder expiration date of 12/31/2069 (Centers for Disease Control and Prevention, 2020). A manufactured date will be on the packaging, but this should not be confused with the expiration date. Additional information will be provided about how to access expiration information for individual COVID-19 vaccines. Meanwhile, the CDC is developing “beyond use date” (BUD) tracker labels to assist clinicians with tracking expiration dates at the point of vaccine administration.
Recommendations
Consider the following recommendations to help prepare for flu and COVID-19 vaccination campaigns.
Plan vaccination campaigns
- Establish plans for flu and COVID-19 vaccination campaigns well before implementation, taking into consideration the following:
- Required infection control measures (i.e., scheduling patients, distancing, using personal protective equipment, cleaning/sanitation procedures) that may slow the vaccination process
- Vaccine storage capacity, required storage equipment, and required temperature monitoring
- Optimal staffing (significant reductions in vaccine errors, invalid doses, and missed opportunities to vaccinate have been documented when including pharmacists on the vaccination team) (Haas-Gehres et al., 2014)
- Anticipated language and communication barriers and how they will be effectively handled
- Create and/or examine protocols (and/or standing orders) for flu and COVID-19 vaccines that will be used during a vaccination campaign; confirm that the protocols include:
- Criteria for screening patients for contraindications and precautions
- Directions for preparing and administering the vaccine, including the dose, any required dilution, vials/containers to use, route of administration, and any special precautions
- Details regarding what (e.g., lot number, expiration date), where (e.g., vaccination record, IIS), and how to document vaccine administration and distribution of the Vaccine Information Statement (VIS) or EUA Fact Sheet
- An emergency protocol to follow if the patient develops an adverse reaction
- Information about reporting adverse vaccine events and errors
- Establish in writing how shortages or exhaustions of flu and/or COVID-19 vaccine supplies will be managed and share this document with all staff working on vaccination campaigns
- Confirm that policies and procedures require staff to verify the patient’s current immunization status (and first dose manufacturer if receiving a second dose of the COVID-19 vaccine) prior to vaccination by checking the patient’s health record, vaccination record, and/or IIS
- Plan to evaluate procedures during the first phase of essential worker COVID-19 vaccination campaigns to assess cold chain management and vaccine storage; vaccine preparation, administration, and documentation; risk-reduction strategies; traffic flow; and infection control measures (Centers for Disease Control and Prevention, 2020)
Safe vaccine storage
- Plan for safe storage of flu and COVID-19 vaccines, including:
- Sufficient cold chain capacity to allow vaccines to be stored separately
- Storage (and distribution/transportation) temperature monitoring at all times
- Monitoring of vaccine expiration dates
- Separate the storage of vaccines and other medications in freezers and refrigerators, bins and containers, and coolers and other temporary storage containers
- If possible, use a separate refrigerator/freezer to store vaccines (no other medications)
- Adult and pediatric formulations of the same vaccine should be separated
- Vaccines with similar names should not be stored in bins or containers next to each other
- Consider purchasing differing age-specific formulations of the same flu vaccine from different manufacturers to help distinguish them
- Ensure that there are no frozen or refrigerated look-alike products (e.g., similar container volume, shape, label) with which the vaccines might be confused
- Sequester neuromuscular blocking agents in a sealed box or a rapid sequence intubation (RSI) kit
- Label the area where vaccines are stored
- Labels may provide additional information unique to each vaccine (Howard, 2020)
- Labels can draw attention to two-component vaccines, reminding staff to mix them (Samad et al., 2020)
- Store vaccines in the original carton, which is fully labeled, to help prevent errors, protect the vaccine from light, and keep the vaccine within its recommended temperature range (Howard, 2020)
Staffing and training
- Staff vaccination campaigns with trained providers who have demonstrated competencies related to the flu and/or COVID-19 vaccine(s) and error-prevention strategies
- Confirm that staff involved in the vaccination campaigns know how to search the IIS, understand the information provided, and how to document immunizations in the IIS
- Verify that staff understand the need for read-back and/or repeat-back of oral communication among colleagues and patients while wearing a mask and/or face shield
Safe vaccine dispensing
- Use commercially available, prefilled syringes of flu vaccines whenever possible
- When space permits, affix auxiliary labels or highlight important label information with a marker for:
- Vaccines with similar names or components, or different age formulations
- Critical label information that is not prominent (e.g., dilution instructions)
- Two-component vaccines, to ensure that they are mixed together prior to administration
- Establish a process to keep two-component vaccines together if storage requirements do not differ
- If barcode technology is used prior to administration, affix a barcode to vaccines or ensure that the manufacturer’s barcode is easily scannable
- Check for expired vaccines prior to a vaccination campaign
Safe vaccine administration
- Label all syringes of prepared COVID-19 (or flu) vaccines using preprinted or blank labels
- Employ barcode verification prior to administration when possible
- Immediately prior to administration, document the vaccine, manufacturer (particularly important for COVID-19 vaccines), lot number, and expiration date in the patient’s electronic health record; document actual vaccine administration afterwards
- Within 24 hours of administering a dose of the COVID-19 vaccine, record it in the IIS and report required information to the relevant state/local public health authority (Centers for Disease Control and Prevention, 2020)
Patient education
- Provide a current VIS (or an EUA Fact Sheet for patients receiving COVID-19 vaccination) to patients or caregivers in their primary languages prior to vaccination (ismp.org/ext/46)
- Incorporate the patient or caregiver into the checking process and show them the vaccine prior to administration
- Educate the patient or caregiver about the need for a second COVID-19 vaccination, if appropriate; if possible, develop a process to send a reminder to the patient
- Provide a completed COVID-19 vaccination record card to every patient or caregiver, which clearly indicates when/if they need to return for a subsequent dose (Centers for Disease Control and Prevention, 2020)
Report adverse vaccine events
- Establish a vaccine safety monitoring system so that adverse events following vaccinations are identified, reported, and investigated; take action to prevent future adverse events
- Report serious adverse effects, multisystem inflammatory syndrome (MIS) in children or adults, and cases of COVID-19 that result in hospitalization or death to the Vaccine Adverse Event Reporting System (VAERS, ismp.org/ext/592) (Centers for Disease Control and Prevention, 2020)
- Report any vaccine errors to the ISMP National Vaccine Errors Reporting Program (ismp.org/VERP)
References
American Pharmacists Association. (2020, November 5). Addressing the COVID-19 crisis: An open forum webinar series for pharmacists. YouTube. https://www.youtube.com/watch?v=ySAjgYYVp6k&feature=youtu.be
Associated Press. (2020, November 12). As COVID-19 vaccine draws closer, states ramp up for biggest vaccination effort in U.S. history. www.ismp.org/ext/590
Blank, C. (2020, November 4). Pharmacy chains report higher than usual demand for flu vaccines. Drug Topics. www.ismp.org/ext/582
Centers for Disease Control and Prevention. (2020, October 29). COVID-19 vaccination program interim playbook for jurisdiction operations. Version 2.0. www.ismp.org/ext/593
Haas-Gehres, A., Sebastian, S., & Lamberjack, K. (2014). Impact of pharmacist integration in a pediatric primary care clinic on vaccination errors: A retrospective review. Journal of the American Pharmacists Association, 54(4), 415–418. https://doi.org/10.1331/japha.2014.13094
Howard, J. J. (2020). Look-alike vaccines: Influenza (Fluarix Quadrivalent) and hepatitis A (Havrix). Veterans Administration Center for Medication Safety. Medication Safety in Seconds, 9(10), 3.
Moderna. (2020, November 16). Moderna announces longer shelf life for its COVID-19 vaccine candidate at refrigerated temperatures [Press release]. www.ismp.org/ext/594
Paris, C., & Hopkins, J. S. (2020, October 21). Pfizer sets up its “biggest ever” vaccination distribution campaign. The Wall Street Journal. www.ismp.org/ext/583
Samad, F., Burton, S. J., Kwan, D., Porter, N., Smetzer, J., Cohen, M. R., Tuttle, J., Baker, D., & Doherty, D. E. (2020, November 5). Strategies to reduce errors associated with 2-component vaccines. Pharmaceutical Medicine [Epub ahead of print]. https://doi.org/10.1007/s40290-020-00362-9
From ISMP Medication Safety Alert! Acute Care Edition, November 19, 2020, Volume 25, Issue 23.
