Five Tips for Smooth Adoption of Safer Enteral Connectors
Nebraska Medicine’s ENFit Conversion Designed to Improve Patient Safety
By Stacie Ethington, MSN, RN-BC
Background
Adverse outcomes caused by enteral tubing misconnections are well documented in The Joint Commission’s Sentinel Event Alert from August 2014 that states “Tubing misconnections continue to cause severe patient injury and death.” To address the issue, in fall 2020 Nebraska Medicine converted legacy enteral tubes and disposables to be compatible with the ISO 80369-3 standard, also known as ENFit®, that is designed to prevent misconnections and disconnections.
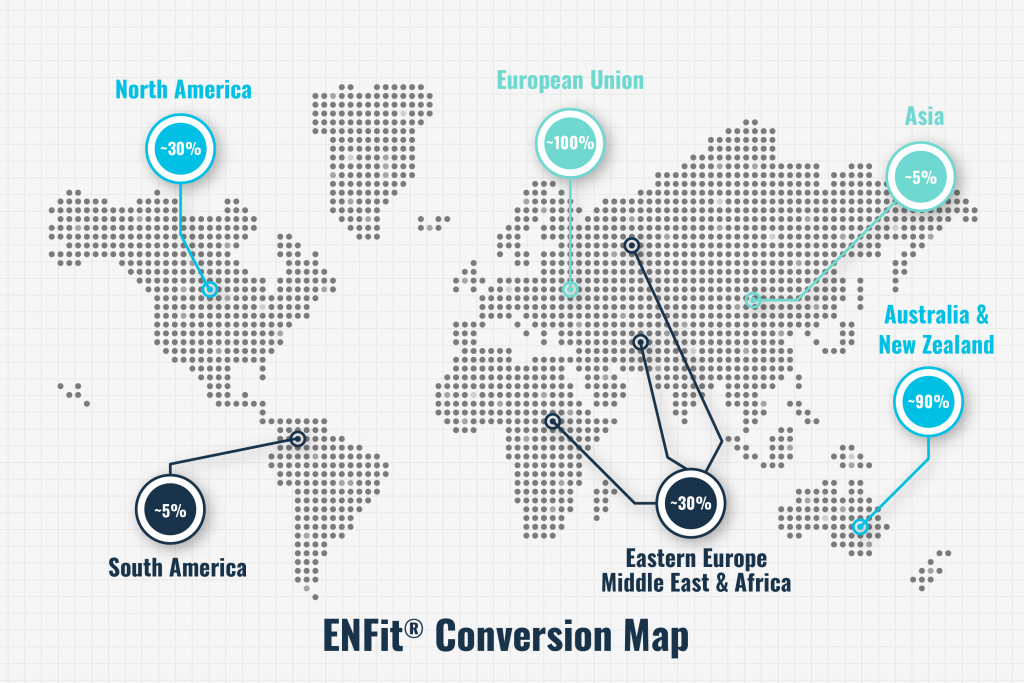
ENFit has been on the market for several years worldwide, and conversions to the ISO standard have been completed in the European Union, Australia, and New Zealand and are underway in Japan and Brazil. In the U.S., nearly half of the top 25 hospitals are now using ENFit, including leaders in patient-centric care such as Mayo Clinic, Sharp Memorial, Banner Healthcare, and Kaiser Permanente. However, the U.S. is lagging behind most other developed countries in adopting the safer connectors.
The effort has been driven by the Global Enteral Device Supplier Association (GEDSA), a nonprofit organization that promotes patient safety worldwide through the adoption of ISO 80369–compliant small-bore medical connectors. Furthermore, the Institute for Safe Medication Practices (ISMP) promotes use of “an oral syringe or an enteral syringe that meets the [ISO] 80369 standard, such as ENFit” as part of its 2020-2021 Targeted Medication Safety Best Practices for Hospitals.
Planning
Nebraska Medicine began its journey toward ENFit conversion in December 2019. An important first step was organizing a project team that included representation from project management, nursing, nutrition, pharmacy, physicians, purchasing, and the supply chain. The project team met with possible vendors over the course of several days. This gave end users the opportunity to get their hands on the products and ask questions related to their areas of expertise. Five vendors were reviewed. The group discussed the pros and cons of each vendor, and those selected were vetted through senior leadership. With their approval, product conversion planning began. The activities that followed included workflow development with the completion of a failure mode and effects analysis, policy revision and creation, physician engagement, education development, and enhancement within the electronic health record (EHR).
Implementation
Prior to conversion, nursing and pharmacy completed educational modules. The video-based nursing module focused on the ENFit connection, medication preparation, connector cleaning, and documentation within the EHR. The PowerPoint®-based pharmacy module focused on medication preparation. Roaming in-services were completed by the product vendors on the days surrounding conversion. A small group of resource nurses served as the experts for frontline staff to contact with issues or questions. Related nursing policies went live the day of conversion.
Lessons learned
- Assess compatibility with NICU enteral pumps
A major lesson learned during the project was that the enteral pumps in the neonatal ICU were not compatible with the vendor chosen for disposables. A pump model upgrade would be needed for our enteral pumps to be functional with an ENFit syringe. Luckily, the enteral pumps were due for replacement within the next fiscal year, so the project team was able to get approval from senior leaders for an expedited replacement plan. The new pumps were implemented in tandem with the ENFit conversion. Having two vendors on-site for go-live support was a challenge to coordinate due to visitor restrictions with COVID-19, but the frontline staff appreciated the “at the elbow” on-site support for conversion. If pump compatibility is a potential issue for your organization, consider evaluating not only if ENFit syringes can be run on your pump, but also what size syringes are compatible. The pump manufacturer should have information regarding which ENFit manufacturer’s syringes are approved for use with the device.
- Ensure adequate supplies of essential equipment
Three key areas to consider:
- 60 mL syringe. During conversion, many of the current legacy products were swapped out for the ENFit compatible version of that product. For example, a 10 mL amber oral syringe would be swapped with a 12 mL ENFit syringe. Following educational virtual calls regarding the conversion, units were given a suggested product list with descriptions. Ordering occurred at the unit level, meaning an ICU manager would determine product and parts for their unit, and the same process would occur on other units. One issue that was quickly identified in the days following conversion was that many nursing units did not have 60 mL ENFit syringes available. A deep dive revealed that 60 mL Toomey syringes still had some indications in patient care, so units did not want them converted. This led to a “miss” with realizing the need for adding the 60 mL ENFit syringe.
- Each organization must make a decision whether adapters will be used during conversion. Since our organization did a full conversion all at once, adapters were necessary. Similar to the issue above, some nursing units did not order enough adapters for conversion. While the vendors identified the need for the availability of the adapter, the volume needed was not well understood.
- Lopez valve. Another supply need that was not appropriately anticipated was the need for an ENFit-compatible Lopez valve. While there are several practice scenarios where these valves were used in relation to enteral tubes, the following section outlines the major need identified.
- Coordinate early involvement of the supply chain
Despite involving members of our supply chain early in our planning process, the lift required for a successful conversion was underestimated. When late needs were identified (60 mL syringe, ENFit compatible Lopez valves, need for Legacy tubes for decompression, etc.), it was difficult to quickly resolve the issues. An important factor here is that we went live during a global pandemic. A debrief has occurred in an effort to promote more comprehensive planning for similar future projects.
- Understand suction/decompression issues for outpatient use
Patients in the inpatient setting could have their ENFit enteral tubes hooked up to wall suction if needed (e.g., by using an ENFit compatible Lopez valve). However, suction and decompression in the outpatient setting has proven to be a challenge. Some outpatients have had large-bore enteral tubes hooked up to gravity bags to allow for decompression. One scenario occurred where a patient was converted to an ENFit compatible tube, but then had issues at home related to leaking, inability of tube to drain, etc. Due to the smaller inner lumen of the ENFit tube, thicker secretions were unable to drain.
Discussions occurred with our vendors and internal provider experts, and ultimately the decision was made to keep some legacy enteral tubes within the organization specifically for this patient population. These tubes are labeled “**FOR DECOMPRESSION ONLY**” within our EHR to prevent any confusion with ordering. Our organization has hope that this patient need is considered as manufacturing of legacy enteral tubes potentially decreases or ceases.
“Due to running two production lines, innovation of new products for issues such as this was held up,” said GEDSA Executive Director Mike Cusack. “With the promise of phasing out legacy from production lines, this has helped to provide the resources needed to create new products that help patients and those who care for them. We have heard such talk of new devices coming to the market soon.”
- Educate community partners
As Nebraska Medicine prepared for go-live, the need for community outreach was evident. Our organization identified that during the transition phase, patients and caregivers might experience variability in enteral access devices requiring ENFit adapters and connectors. ENFit enteral tubes must be accessed with ENFit syringes or tubing sets. Should a patient with an ENFit enteral tube present to an outside organization that does not have ENFit supplies, the caregivers will not be able to access the enteral tube. If a patient with a legacy tube arrives at an organization that has converted to ENFit, an adapter can be placed on the ENFit syringe or tubing set so that the enteral tube can be accessed.
In February 2020, Nebraska Medicine sent out letters to many of our community healthcare partners regarding our intent to convert to ENFit later in the year. Within that letter, resources were shared for those that had not had exposure to the initiative. Unfortunately, post go-live we learned that outside facilities did not always have the supplies needed to care for our patients discharged with ENFit. We coached them if they reached out, and ultimately decided to send out a second letter to the community healthcare partners to let them know our conversion had been completed; we again provided resources within the letter.
As another method to ensure that our patients could safely transition their care, we had discharge orders built within our EHR that prompt the discharging nurse to provide the patient with an appropriate syringe and/or adapter based on the type of enteral tube the patient is being discharged with.
Conversion resources
Our nursing staff was positive about the transition. “The extensive planning required for a safe and successful conversion has been well worth it,” one nurse commented. “We have experienced much satisfaction from our nursing staff.” Another nurse commented how ENFit improves patient safety while streamlining workflows. “I love the ENFit adapters because they lock onto the tubing so well that I do not have to worry about the tubing detaching and making a mess,” the nurse said. “It takes one less worry away from my workflow and saves me time with no mess.”
If your organization has not yet converted, there are several resources available. GEDSA’s website has many excellent articles, presentations, and tools, such as a transition checklist. Many of the enteral device suppliers have resources on their websites as well. Another invaluable tool is the ability to network with other organizations that are going through the same process so that lessons learned can be shared.
Stacie Ethington, MSN, RN-BC, is a medication safety nurse specialist at Nebraska Medicine in Omaha.
