Drug Shortages Continue to Compromise Patient Care

By the Institute for Safe Medication Practices
An exhaustive account of frustrations, difficulties, misspent resources, and safety concerns came across loud and clear from respondents who participated in ISMP’s August through October 2017 national survey on drug shortages. Most respondents to the survey confirmed that drug shortages during the six months prior to the survey continue to be a daily struggle, involving an increasing number of lifesaving drugs without a viable alternative and lasting longer than ever before. They suggested that providing safe and appropriate drug therapy has become extremely challenging during shortages and has led to numerous instances of unsafe practices, compromised care, and potentially harmful errors.
Many respondents noted that the state of drug availability in the U.S. is unacceptable. Some believed that inadequate planning during recent drug company mergers has led to many of the recent drug shortages. Others left angry comments blaming the drug manufacturers for limiting supplies to increase demands and bolster profits, noting that products in short supply that return to the market are often more costly than before the shortage. But most of the respondents clearly struggled to understand why the drug shortages continue to occur at an alarming rate that makes it nearly impossible to provide safe, high-quality patient care in a fiscally responsible manner. Details from the survey follow. Please note: Some responses to this survey occurred before, and some after, Hurricane Maria hit Puerto Rico in September 2017, which significantly worsened certain drug shortages.
Respondent profile
Nearly 300 respondents completed our survey on drug shortages, including pharmacy directors (37%), pharmacy managers or assistants (26%), pharmacy purchasing agents (21%), clinical/staff pharmacists (8%), pharmacy technicians (3%), medication safety officers (2%), and others (3%). Almost all respondents practiced in a hospital setting, including community hospitals (56%), teaching hospitals (21%), critical access hospitals (9%), and specialty hospitals (3% pediatrics, 3% long-term acute care, 4% other).
Drugs involved and frequency of shortages
Over half (55%) of all respondents reported that more than 20 drugs were involved in shortages during the six months prior to the survey. However, differences were seen among respondents from various practice settings. For example, 19% of respondents from critical access hospitals reported experiencing shortages with more than 20 drugs during this time, compared to 73% of respondents from teaching hospitals and 62% from community hospitals. Most were affected by at least one drug shortage daily, particularly those from specialty (80%), community (77%), and teaching hospitals (73%).
Shortages were reported across all treatment categories. Over two-thirds of respondents reported shortages that impacted emergency care (87%), anesthesia care (85%), pain management (81%), infectious disease treatment (71%), and cardiovascular care (68%). More than half of the respondents experienced shortages that impacted parenteral nutrition (55%), while one-third involved obstetrics/gynecology (33%) and hematology/oncology (33%) service lines. Vital drugs that impacted many other service lines were also reported, including neurology (18%), allergy/asthma care (15%), psychiatry (10%), endocrinology (10%), and ophthalmology (5%). Five percent of respondents reported intravenous (IV) fluids in short supply, affecting all service lines (which worsened after Hurricane Maria).
Advanced notification about shortages
Few respondents reported consistently receiving advanced notifications from drug manufacturers, wholesalers, distributors, group purchasing organizations, or the U.S. Food and Drug Administration (FDA) about impending drug shortages (12%), their causes (13%), or their duration (11%). However, improvements were observed in our 2017 survey when compared to a similar survey we conducted in 2010 (Institute for Safe Medication Practices, 2010). In 2010, 84% of respondents said they never or rarely received advanced notifications about shortages, their causes, or duration. In 2017, notifications about drug shortages were more frequently reported, although 38% still said they never or rarely received this information.
Interdisciplinary tension
Approximately two-thirds (62%) of all respondents reported that patients, medical staff, hospital leadership, nurses, and/or staff from other clinical departments frequently or always expressed frustration with pharmacists or pharmacy staff members because of drug shortages. Few differences were seen among respondents from different settings.
Actions to address drug shortages
Respondents reported taking many resource-intensive actions to reduce the impact of drug shortages on patient safety and to ensure patients receive the required treatment.
Secure and maintain products. Most respondents reported actions that have significantly increased drug costs because of drug shortages. At least 90% of respondents reported adding backup inventory for critically important drugs in short supply, changing par levels, purchasing excess inventory from a wholesaler, and/or purchasing a more expensive brand, generic, or therapeutic alternative product from a wholesaler. More than half of the respondents purchased more expensive products from a new distributor (67%) or an outsourcer (58%). Fifteen percent admitted to purchasing drugs in short supply at great cost from a secondary gray market, which represents a decrease when compared to a 2011 survey (Institute for Safe Medication Practices, 2011) in which half of the respondents admitted to purchasing medications from the gray market. To secure a needed medication, respondents also said they borrowed or purchased it from another health system, purchased it directly from the manufacturer or in different strengths/concentrations, or compounded it.
Limit or extend drug use. Ninety-four percent of respondents reported rationing or restricting drugs in short supply. Examples included establishing criteria for using products, restricting access to drugs via override in automated dispensing cabinets (ADCs), and providing kits for emergency drugs. Thirty percent of all respondents said they have used a drug in short supply outside its specific labeling to help extend its use, such as keeping expired products (without FDA-extended dating) in code carts.
Communicate and educate. Almost all respondents (97%) reported devoting resources to keep the medical staff informed about drug shortages. A vast majority had also established regular meetings with pharmacy staff to address the shortages (79%) and devoted resources to staff education about the safe dosing of alternative drugs (80%).
Adverse patient outcomes
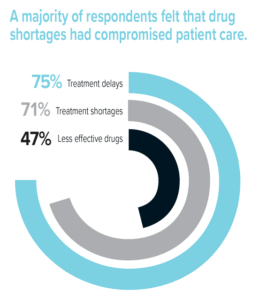
A majority of respondents felt that drug shortages had compromised patient care. Most (71%) were unable to provide patients with the recommended drug or treatment for their condition due to shortages, and nearly half (47%) thought that this resulted in patients receiving a less effective drug. Also, three-quarters (75%) of respondents stated that patient treatments had been delayed because of drug shortages. One example involved a delay in treating sepsis and acidosis using sodium bicarbonate that may have contributed to a patient’s death. An additional 5% of respondents reported other types of adverse outcomes related to drug shortages, such as increased pain or discomfort during a procedure due to the unavailability of a required analgesic or sedation agent.
 Adverse impact on organizational resources
Adverse impact on organizational resources
Although not directly queried in the survey, more than one-third of all respondents took the time to provide comments voicing their anger and frustration with the extensive human and financial resources that have been required to manage drug shortages. Many said they needed full-time staff to manage drug shortages and felt the tasks associated with this process had cut into the time normally devoted to patient care and medication safety, introducing risk, and contributing to errors. Many respondents also provided examples of how recent drug shortages have led to unsafe practices that have increased the risk of an error. Examples included:
>Dispensing medications in vials to patient care units so they can be prepared and administered via IV push administration; these medications were previously available in premixed containers or mixed in the pharmacy in small-volume containers
>Administering IV push medications rapidly when they should be administered more slowly via a syringe pump
>Diluting or reconstituting medications in saline flush syringes on patient care units due to shortages of normal saline
>Compounding products in the pharmacy and in the operating room that were previously available as premixed solutions or injectables
>Providing medications in concentrations that differ from what was typically used for direct injectables, or for compounding products according to standardized formulas, which are then no longer accurate
Errors due to drug shortages
Similar to our 2010 survey, nearly a quarter (21%) of all respondents in our 2017 survey were aware of the occurrence of at least one medication error related to a drug shortage in the six months prior to the survey. Respondents provided descriptions of close to 100 errors; most (67%) were associated with the wrong dose or concentration. Examples are provided in Table 1. Comments by some respondents who were not aware of errors due to drug shortages suggest they may occur but are not being reported. 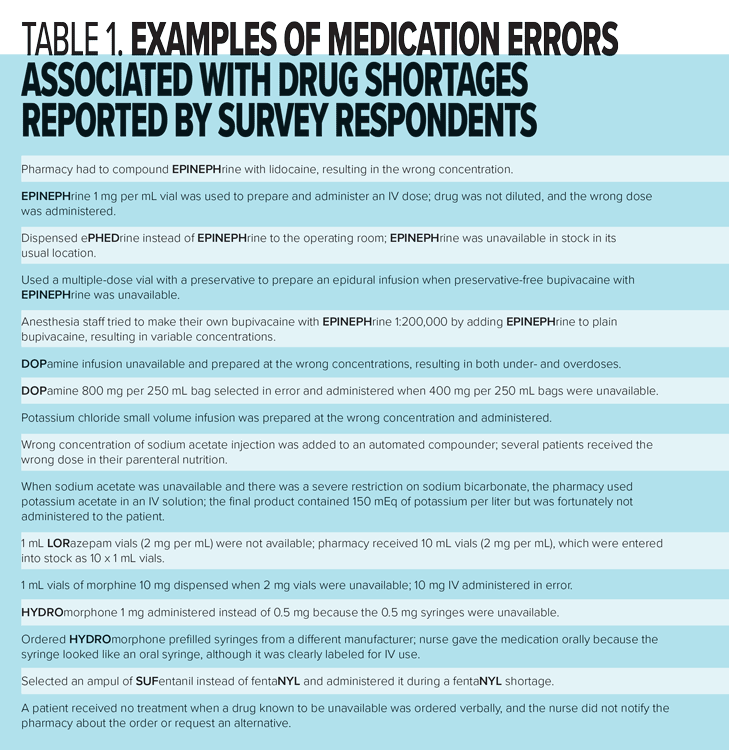
Breaches in drug purchasing or allocation policies
Approximately one in six (17%) respondents were aware of breaches in drug purchasing or allocation policies. The most frequently cited breaches included using drugs supplied in emergency carts or kits for non-emergencies, unauthorized drug purchases, buying excessive amounts of drugs associated with impending shortages, hoarding of drugs, removing a drug in short supply from an ADC via override despite restrictions, using single-dose vials as multiple-dose vials, and purchasing sterile products compounded from non-sterile ingredients from compounding pharmacies without evaluating the risk. Despite these breaches, improvements were observed when compared to our 2010 survey in which more than half of respondents reported breaches such as the hoarding of drugs.
Conclusions
Our national survey suggests that the impact of drug shortages continues to take an enormous toll on healthcare providers and patients. The staff hours alone spent on planning for the shortage; educating staff; restocking and barcoding the alternative products; dealing with secondary market vendors; prescribing, preparing, and administering unfamiliar alternative products; and fielding questions consumes a large portion of the health professionals’ time, stealing valuable resources from clinical activities. Overall, survey respondents conveyed a real sense of crisis, frustration, and anger associated with the ongoing drain of resources and threats to patient safety that the shortages impose.
Numerous organizations and governmental agencies have been working steadily to provide oversight regarding the availability of pharmaceutical products, develop more effective early warning systems for impending shortages, keep clinicians informed about shortages and potential alternatives, and reduce the overall adverse effects of drug shortages. For example, the American Society of Health-System Pharmacists (ASHP), ISMP, and other organizations recently met to develop a congressional call to action to address the causes of drug shortages. The work is slow but ongoing.
Multiple resources are available to organizations that must manage this complex problem, guided by pharmacy leadership, including the following:
>ASHP Resource Center: www.ashp.org/shortages
>FDA Drug Shortages Page: https://www.fda.gov/Drugs/DrugSafety/DrugShortages/default.htm
>U.S. Department of Health and Human Services’ Office of the Assistant Secretary for Preparedness and Response: https://asprtracie.hhs.gov/scarce-resources
>Centers for Disease Control and Prevention guidance on vaccines in short supply: https://www.cdc.gov/vaccines/hcp/clinical-resources/shortages.html
>American Society for Parenteral and Enteral Nutrition guidance on shortages with parenteral nutrition components: http://www.nutritioncare.org/Guidelines_and_Clinical_Resources/Product_Shortages/Product_Shortage_Management
Preparation, standardization, communication, and monitoring is paramount to safely manage drug shortages. Although it may be impractical to prepare for every potential drug shortage, proper planning can minimize the adverse effects on both patients and providers. Also, be sure to update and standardize any processes associated with alternative medications and communicate information to clinicians about the steps taken to limit or extend products in short supply. Utilize error and adverse event reporting systems, as well as focus group meetings, discussions during pharmacy rounds, or other means, to learn about hazardous conditions, close calls, and adverse events associated with drug shortages so actions can be taken to limit further risk and harm.
This column was prepared by the Institute for Safe Medication Practices (ISMP), an independent, charitable nonprofit organization dedicated entirely to medication error prevention and safe medication use. Any reports described in this column were received through the ISMP Medication Errors Reporting Program. Errors, close calls, or hazardous conditions may be reported online at www.ismp.org or by calling 800-FAIL-SAFE (800-324-5723). ISMP is a federally certified Patient Safety Organization (PSO), providing legal protection and confidentiality for patient safety data and error reports it receives. Visit www.ismp.org for more information on ISMP’s medication safety newsletters and other risk reduction tools. This article appeared originally in the January 11, 2018 edition of the ISMP Medication Safety Alert!
References
Institute for Safe Medication Practices (2010, September 23). Drug shortages: National survey reveals high level of frustration, low level of safety. ISMP Medication Safety Alert!
Institute for Safe Medication Practices (2011, August 25). Gray market, black heart: Pharmaceutical gray market finds a disturbing niche during the drug shortage crisis. ISMP Medication Safety Alert!
