Applying the IHI Global Trigger Tool to Pediatric and Special Needs Populations
July/August 2011
Applying the IHI Global Trigger Tool to Pediatric and Special Needs Populations
Gillette Children’s Specialty Healthcare (Gillette) provides specialized healthcare services to individuals with disabilities, with a primary focus on children. Serving a wide geographic region, Gillette’s programs focus on the needs of patients with cerebral palsy, spina bifida, neuromuscular diseases, brain and spinal cord injuries, and complex neurological, craniofacial, and orthopedic conditions. In 2009, Gillette performed 3,320 surgical procedures and provided 2,149 inpatient admissions.
Gillette prioritizes key quality improvement and patient safety initiatives each year, resulting in measurable improvements. In 2005, Gillette began adopting the principles of reliability science to further its efforts in quality. Preventing events requires careful analysis of the processes related to adverse events, which led Gillette to consider how to confirm the integrity and completeness of their adverse event data. According to the Institute for Healthcare Improvement (IHI), public health researchers report that 10 to 20% of errors are reported using voluntary reporting systems (Griffin & Risser, 2009). As Gillette utilizes a voluntary reporting system supplemented by record review, evaluating the effectiveness of the adverse event reporting systems was essential.
The Institute for Healthcare Improvement developed the Global Trigger Tool for measuring adverse events in 2003. A comparison of the indicators on the Global Trigger Tool to the current reporting criteria utilized by Gillette demonstrated significant consistency between the criteria of the two methods. However, as Gillette provides specialty services primarily to pediatric patients, many of whom have uncommon diagnoses, testing the applicability of the Global Trigger Tool was of primary importance. To reliably utilize and test the Trigger Tool, Gillette joined a learning collaborative with the Institute for Healthcare Improvement.
Two primary results were anticipated from this project. First, this project would evaluate the applicability of a modified IHI Global Trigger Tool for the identification of adverse events in the primarily pediatric specialty population served by Gillette. Second, by simultaneously utilizing the voluntary and record review event reporting processes and the IHI Trigger Tool, a comparison of methods for the collection of adverse event data could be completed.
Rationale
Studies of error reporting systems reveal individuals directly involved in care processes detect approximately 75% of errors (Reason, 1990). However, a number of factors influence whether these same individuals then report the errors, impacting the effectiveness of error reporting systems. Trust, blame, confidentiality, and other incentives and disincentives influence reporting behavior (Bagian et al., 2001; Oken et al., 2007; Sorra et al.; 2008 Moumtzoglou, 2010). Recent studies evaluating the performance of event reporting systems report differences in the identification of patient safety concerns by systematic reporting methods as compared to voluntary reporting methods (Silas & Tibballs, 2010; Sari et al., 2007). One method, the trigger method, which uses a prompt or signal to promote investigation, has been shown to be an effective systematic method (Rozich et al., 2003; Resar, 2006; Sharek et al., 2006; Takata et al., 2008). Classen et al. (2011) in a recently published study found the use of the Global Trigger Tool (GTT) developed by IHI identified as least 10 times more significant events than either a voluntary reporting method or the Patient Quality indicators developed by the Agency for Health Care Research and Quality.
Methods/Implementation
Evaluation Team
Beginning in April 2009, two registered nurses from the Quality Improvement Resources Department at Gillette, experienced in clinical and quality improvement activities, participated in education sessions on the IHI Global Trigger Tool and its use. The education as well as the ongoing participation in the learning collaborative assured process reliability.
The CEO served as the project champion while the Patient Services Quality and Safety Committee reviewed the data and data analysis. The activities and results related to this project were kept separate from the data on adverse events collected by voluntary reporting and chart review.
Measurement Activities
As the IHI Global Trigger Tool was designed for use with adults, modifications were made prior to implementation. Triggers not applicable to the patient population or services were removed from the tool to simplify the tool and ease the utilization. These included triggers related to perinatal and emergency department services, dialysis, and troponin level. Also removed from the tool were anti-emetic use, Benadryl/diphenhydramine use, vitamin K administration, and imaging obtained intra-operatively or in the PACU because they capture a planned aspect of care, which is common for Gillette’s patient population. Infection of any kind was modified to trigger healthcare associated infection, and decubiti was changed to pressure ulcer to better align with the language in use in the organization. Population-specific triggers added to the tool included rash, hyperkalemia, and use of sodium polystyrnen (Kayexalate).
Beginning in April 2009, the two reviewers independently screened records using the modified tool and following the IHI screening processes. The criteria for inclusion used for this project consisted of:
- inpatient admission for acute care need (rehabilitation stays excluded),
- length of hospital stay of 3 or more days,
- inpatient documentation completed and record coded,
- patient age 10 years and older.
Every 2 weeks, 10 records were randomly selected from those meeting the criteria. Over 24 weeks, 82 medical records were reviewed by each of the two separate reviewers using the IHI Global Trigger tool (GTT). For each record, in the time allowed by the IHI protocol (20 minutes), the clinical reviewers identified all triggers per IHI protocol and assigned harm to each trigger identified. The definition of harm utilized for this project was any unintended physical injury that resulted from or was contributed to by medical care and that required additional monitoring, treatment, hospitalization, or that resulted in death.
Results
The average length of stay of the evaluation group was 7 days, which compares to the overall hospital average length of stay of 5 days. The range of age of the patients was 10 to 30 years as compared to the range of patients served in 2009 of 0 to 58 years. Forty-eight percent of the records reviewed were of patients 21 years of age and younger.
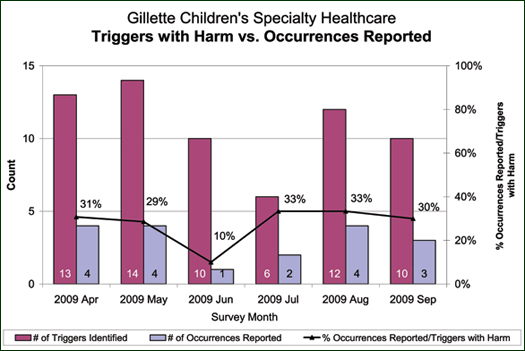
In the 82 records, 114 triggers were identified. Of those, 55 separate adverse events with harm were identified, 22 of which were reported through the voluntary reporting system.
An analysis of the 33 events with harm identified through the GTT, which were not identified through the voluntary reporting system, demonstrated the known difference in criteria and the weakness of a voluntary reporting system. Twenty of the 33 adverse events with harm, or 60.6%, identified through the GTT method, were events not reportable under the current voluntary reporting criteria. This included criteria such as UTI, decreased sensation with epidural pain control, or post-operative nausea. Thirteen, or 39.4%, of the events identified only through the GTT process were events that met the reportable criteria under the voluntary reporting system and yet were not reported. Events identified through the GTT process included a pressure ulcer, a transfer to the PICU, a readmission, a return to surgery, a re-intubation, two surgical injuries, a condition change requiring the assistance of the emergency response team, a CSF leak, a vaginal tear secondary to Foley placement, a fall, wound dehiscence, and over-sedation related to medication administration. All of the events identified through the voluntary reporting system were also identified using the GTT method.
Discussion
This project compared the Global Trigger Tool with a voluntary reporting system in an organization with a culture that encourages responsible reporting to an established screening system. The modified IHI GTT identified a substantial number of adverse events with harm that were not identified within the voluntary reporting system. While known differences in selected criteria existed, the IHI GTT effectively identified adverse events within a specialty population and for children 10 years and older.
Based upon the results of this project, several opportunities exist to improve the reporting systems and ultimately the data available to redesign systems and processes. In several cases, the IHI GTT system utilized different criteria from those utilized in the voluntary reporting system. An analysis of the differences, therefore, may reveal opportunities for modification to the reporting or screening criteria and ultimately to improved data collection. The incorporation of a systematic screening process using triggers into the adverse event identification systems will strengthen the data available. However, the time and personnel commitments to record screening were significant and are an important consideration for adoption. Incorporating screening triggers into electronic documentation offers the potential of improving data collection and using the clinical reviewers’ time and expertise more effectively.
Summary
Creating an environment for the provision of safe, quality care requires careful attention to many factors. Designing, constructing, and evaluating the methods by which data are collected and analyzed enhances the ability of an organization to identify and address patient safety needs. Systematic approaches to the capture of adverse events increase the capture rate and therefore a more reliable data set. Coupled with careful categorization and analysis, this method is a key resource for quality improvement. Improving the systems and approaches based upon careful analysis of the data to reveal new understandings is one that meaningfully advances safe patient care.
Christine Milbrath is associate professor and director of health science programs for the College of Nursing and Health Sciences at Metropolitan State University in St. Paul, Minnesota. She teaches quality, safety, health policy, and leadership and serves as a patient care consultant for Gillette Children’s Specialty Healthcare. Milbrath may be contacted at Christine.milbrath@metrostate.edu.
Gail Pries is manager of quality improvement and regulatory affairs at Gillette Children’s Specialty Healthcare. She may be contacted at gpries@gillettechildrens.com.
Pamela Howard and Gayle Huseth are clinical reviewers at Gillette Children’s Specialty Healthcare.
References