Alarm Fatigue Hazards: The Sirens Are Calling
May / June 2012
![]()
Alarm Fatigue Hazards: The Sirens Are Calling
 Nurses often compare their patient care environments to a casino or carnival; a cacophony of sounds and little distinction of where these sirens originate and what they mean. Clinicians cope by turning alarms down or off to create a more tolerable environment for themselves and their patients. Unfortunately, all too often this results in harm to the patient. A family in Boston recently accepted an $850,000 settlement in a case where alarms were defeated, which underscores the tragedy and costs associated with this technology hazard (Kowalczyk 2011). Cases like this one are occurring in many states. In a recent survey by the Healthcare Technology Foundation (2011), one in five respondent hospitals identified an avoidable adverse event due to alarms in the past two years. ECRI Institute has rated alarm hazard as the number one or two patient safety issue in its “Top 10 Technology Hazards” since initiating the annual list (ECRI Institute, 2011). Review of the FDA Maude database for 2011 for the class of arrhythmia-detecting monitors indicated 550 reported alarm hazards, resulting in 35 deaths. Experts believe these data under-report the actual incidents of patient harm due to alarm fatigue. Professional organizations are collaborating to address this serious patient safety problem. The Association for the Advancement of Medical Instrumentation (AAMI) convened a summit in 2011 jointly sponsored by the FDA, ECRI Institute,
Nurses often compare their patient care environments to a casino or carnival; a cacophony of sounds and little distinction of where these sirens originate and what they mean. Clinicians cope by turning alarms down or off to create a more tolerable environment for themselves and their patients. Unfortunately, all too often this results in harm to the patient. A family in Boston recently accepted an $850,000 settlement in a case where alarms were defeated, which underscores the tragedy and costs associated with this technology hazard (Kowalczyk 2011). Cases like this one are occurring in many states. In a recent survey by the Healthcare Technology Foundation (2011), one in five respondent hospitals identified an avoidable adverse event due to alarms in the past two years. ECRI Institute has rated alarm hazard as the number one or two patient safety issue in its “Top 10 Technology Hazards” since initiating the annual list (ECRI Institute, 2011). Review of the FDA Maude database for 2011 for the class of arrhythmia-detecting monitors indicated 550 reported alarm hazards, resulting in 35 deaths. Experts believe these data under-report the actual incidents of patient harm due to alarm fatigue. Professional organizations are collaborating to address this serious patient safety problem. The Association for the Advancement of Medical Instrumentation (AAMI) convened a summit in 2011 jointly sponsored by the FDA, ECRI Institute,
American College of Clinical Engineering, and The Joint Commission to bring stakeholders together to address this complex problem (AAMI, 2011).
Alarm fatigue happens when too many alarms occur in a clinical environment, causing clinicians to miss true clinically significant alarms. Users report that more than 350 alarms per patient per day result from monitoring systems alone in some acute care environments, but less than 5% of these alarms require clinical intervention to avoid patient harm (AAMI, 2011). Nuisance alarms represent the 95% of alarms that do not require a clinical intervention. Reducing the overall occurrence of nuisance alarms is essential in creating and maintaining a safe clinical environment. Furthermore, solving this vexing problem is essential to improve patient safety systems. Experts in rapid response agree that improving the early detection of deteriorating patients is required to make rapid response systems an effective tool. A recent consensus article recommends continuous vital signs monitoring as a mechanism for strengthening rapid response systems, but only if nuisance alarms are addressed (DeVita et al., 2010).
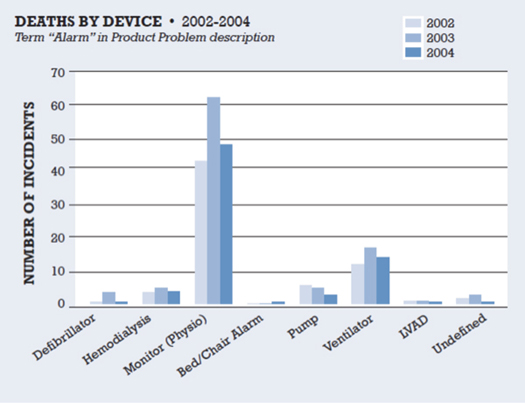
Alarms originate from a plethora of medical devices, each with its own unique characteristics, few of which are coordinated in the clinical environment. The distribution of alarms is care-unit specific and dependent on the types of devices, vendors, and alarm configuration settings. Figure 1 illustrates the distribution of alarms by device associated with death in the FDA Medical Device Reporting (MDR) database between 2002 and 2004 (ACCE Health Technology Foundation, 2006).
Since this survey was published, more devices have been added to the clinical environment, which has added complexity to the clinical setting. One example is out-of-bed alarms, which warn nurses of the potential for patient falls.
Figure 1. Impact of Clinical Alarms on Patient Safety
Reprinted with permission from the ACCE Healthcare Technology Foundation (2006).
Alarm Classifications
Addressing alarm fatigue is a challenging human factors problem involving devices, systems, and workflow components. A starting point is to classify the types of alarms and then decompose each of these classes further to address root causes. The following classifications were developed based on consideration of clinician workflow.
False alarms
False alarms are generated due to bad or missing data. False alarms are often caused by patient motion, poor sensor placement, intermittent cables, and limitations in the device alarm detection algorithm. Manufacturers have advanced the design of sensors and detection algorithms, but if the medical devices are not properly applied or maintained, false alarms will persist. Biomedical engineering departments can play a significant role in reducing false alarms through routine testing and inspection of medical devices. Purchasing departments need to assess the savings of lower cost or reprocessed sensors against their performance to ensure lower prices do not result in a higher incidence of false alarms. Participants in the AAMI Summit advised the daily replacement of ECG electrodes to reduce nuisance ECG alarms.
Non-actionable alarms
Non-actionable alarms are true alarms that do not require a clinical intervention or are the result of intentional actions. They distract clinicians’ attention needlessly and therefore are a nuisance. In most cases these short duration alarms correct themselves. Examples are short duration low-oxygen saturation or heart-rate alarms. Both of these cases occur frequently with the alarming vital sign returning to a normal range within a few seconds. Logging these changes may be important for patient care plans, and repeated patterns may be a precursor to an emerging alarm condition, but audible annunciation does not necessarily require an immediate response each and every time it occurs. Other non-actionable alarms may require additional context. Intentional activities by trained clinicians at the bedside can generate non-actionable alarms. For example, suctioning an intubated patient can trigger a ventilator alarm that does not require changing or modifying settings, yet the occurrence of the audio alarm adds to environment stimulation for all clinicians within the vicinity of the device. Non-actionable alarms are the major contributor to nuisance alarms. Adding audio alarm delays can substantially reduce nuisance alarms.
Actionable alarms
Actionable alarms require timely intervention to avoid an adverse event. Defining actionable alarms requires identifying the most important attributes of the alarm that have a high probability of causing harm. Time to response must also be considered. A common trait of actionable physiologic alarms is persistence. Most high or low sustained vital signs are examples of actionable alarms. Exceptions to this rule are life threatening alarms such as asystole or apnea. Infusion pumps implement alarms as workflow reminders or provide warning of inappropriate drug delivery settings. Examples include Infusion completion, positional IV catheter, and KVO rate. These alarms are not persistent dependent but may benefit from other contextual information to separate non-actionable events. Other medical devices possess different attributes, each with unique conditions that can lead to harm. Separating actionable and non-actionable alarms requires identification of the contextual conditions that must persist in order for an avoidable adverse event to occur. Setting overly sensitive conditions contributes to nuisance alarms. Alternatively, setting too liberal conditions may produce false negative events.
Strategies for Reducing Nuisance Alarms
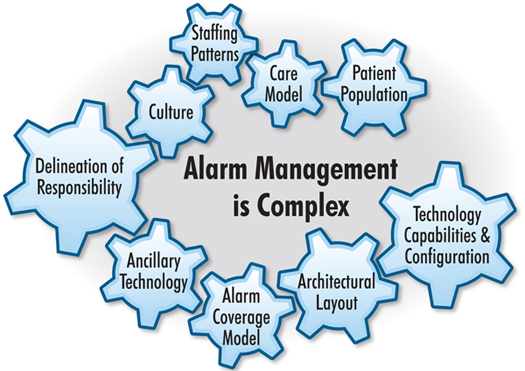
Reducing nuisance alarms requires a multidisciplinary approach including partnerships with device manufacturers. Each medical device has its own unique profile of alarm configurations and settings. Although there is an international standard for alarms—ISO 60601-1-8—not all device manufacturers adopt the standard and each has developed their alarms with minimal consideration of other devices sharing the clinical environment. Stakeholders in solving alarm fatigue come from the clinical, technical, and IT community. Clinicians are in the best position to define acceptable alarm configuration settings. Clinical engineers and biomedical technicians can inform the team of the trade-offs between alarm sensitivity and specificity within the constraints of the medical device. IT engineering can provide support for remote alarm annunciation if such capabilities are required. Figure 2 illustrates a framework developed by ECRI Institute (2011), in which a team can identify underlying causes of nuisance alarms.
Figure 2. Framework for Alarm Management
ECRI Institute (2011); Reprinted with permission.
Evidence-Based Alarm Optimizations
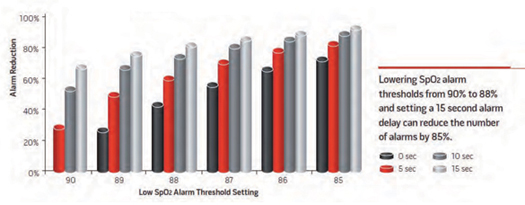
The highest contributor to false and nuisance alarms is continuous vital signs monitors. Most of these devices are connected to central servers where data is collected and stored for trend displays. This data can be analyzed retrospectively to determine optimized alarm configurations and to minimize non-actionable nuisance alarms. Analysis can implement scenarios to determine the effect of configuration settings on the frequency that a given alarm can occur. An example of retrospective analysis of pulse oximetry is shown in Figure 3 (Welch, 2011). Reducing the low SpO2 alarm from 90%, no-alarm delay to 88%, and a 15-second alarm delay reduced the occurrence of low SpO2 alarms by more than 80%.
Figure 3. Reduction in Alarm Frequency
Welch, 2011; © Horizons, Allen Press Publishing Services. Reprinted with permission.
An alternative approach is to study the before-and-after effects of configuration setting. A team at The Johns Hopkins reviewed the incidence of alarms on a progressive care unit, made alarm configuration changes, and studied the results (Graham & Cvach, 2010). They also identified root causes of alarms independent of the medical device and made workflow changes that further reduced nuisance alarms. They reported a 68% reduction in nuisance alarms through improvements in education, daily changing of ECG electrodes, and modest changes to device alarm configuration.
A third approach is through an observational study. Researchers at University of Utah directly observed all device alarms occurring in the environment of care within an ICU (Gorges, Markewitz, and Westenskow, 2008). Two hundred observational hours yielded 1,214 alarms or 6.07 alarms per hour (145 per patient day) for which 5.7% were actionable patient-related alarms and 17.7% were actionable technical alarms. Researchers concluded that an alarm delay of 19 seconds would reduce alarm occurrence by 67%.
These approaches to alarm fatigue reduction are all data driven and specific to the environment of care, workflow, and devices. Conclusions and recommendations from these studies may not produce the same results in other hospitals. However, the methodologies are sound practices within the principles of continuous improvement.
Impact of Reduced Nuisance Alarms on Patient Safety Outcomes
Medical devices alarms are intended to alert clinicians that a patient is outside the normal operating conditions of a medical device. Alarms should warn the clinician of an unsafe condition only when such a condition exists and in sufficient time to rescue the patient. The application of medical devices therefore should contribute to improving patient safety. A cross-functional team at Dartmouth Hitchcock Hospital identified the need for early detection of the deteriorating patient on the general care floor (Taenzer et al., 2010). Team members identified alarm management as the most important attribute to ensure a sustainable success to the proposed system. They recognized that the general care setting needed high-specificity alarms to notify a clinician of a rescue event (surveillance) versus ICU alarms that warn the clinician of a change in physiologic condition. Therefore, surveillance settings for the general care unit were set at broader thresholds, with a 30-second delay before a pager notification was sent to the nurse assigned to the patient. They implemented a policy of continuous monitoring of oxygen saturation and pulse rate for all patients admitted to the surgical general care floor under study. After 11 months of study, they measured a 67% reduction in rapid response calls and a 48% reduction in transfers to the ICU. Alarm rates averaged 4 alarms per patient per day, which included both physiologic alarms (SpO2, PR) and technical alarms. Mortality improved but was not statistically significant because of the power of the study population. The authors estimated an annual ICU bed savings of 136 days. These results support clinical, financial, and operational improvement and would not have been possible without a focus on alarm management.
Recommendations
The 2010 AAMI Alarm Summit has been summarized in a publicly available monograph (www.AAMI.org). The top ten recommended actions focus on three themes:
• Identify and support a multidisciplinary team with clinical leadership support.
• Establish a data driven, continuous improvement process that optimizes alarm thresholds and delays.
• Mandate alarm management training for all clinical operators.
Beyond these user-based recommendations, more can be done by manufacturers and regulatory authorities. Manufacturers have not completely embraced the IEC 60601-1-8 standard that defines how audio and visual alarms are annunciated. Manufacturers use different terminology to describe alarm behavior. These differences confuse end users when going from one care area to another or one hospital to another where brands may differ. The lack of common terminology, audio and visual behavior, and prioritization rules results in training challenges and low learning retention. Regulatory authorities need to place more emphasis on compliance to the standard as a condition for market clearance. The standard also needs to be expanded to cover more types of medical devices. The clinical environment rarely involves a single alarm-generating device. Clinicians differentiate alarms primarily by the audio characteristics. As new devices are introduced, regulatory authorities need to ensure devices not covered by the standard do not conflict with already agreed upon alarm profiles.
End user surveys consistently report the need for smarter alarm systems that combine signals from several sources into a single index of patient condition. Such systems are emerging but face higher regulatory barriers to clearance due to the lack of consistent guidance on the criteria to establish safety and efficacy. Regulatory authorities need to develop a “least burdensome” guidance to expedite innovations in smart alarm technologies.
Alarm hazards are a growing patient safety issue. The Joint Commission is currently conducting a survey, the results of which may impact how future accreditation audits are conducted. Hospitals and patient safety professionals should be proactive by initiating teams that address alarm fatigue throughout their institutions. Further research is needed in integrating data from various medical devices to provide useful information and decision support algorithms. Finally, medical devices are tools used by clinicians. Investment in training users allows these tools to be optimized for patient safety.
Jim Welch is vice president of quality systems and regulatory and clinical affairs at Sotera Wireless. His prior experience includes 17 years as a hospital-based clinical engineer, 10 of which were at Massachusetts General Hospital, where he created one of the first WiFi-based general care floor multi-parameter monitoring systems. Welch also has held executive, engineering, and marketing positions with companies focused on developing and deploying wireless patient safety solutions for the hospital general care wards. In 2011, Welch received the AAMI Foundation/Institute for Technology in Health Care Clinical Application Award for his collaboration with hospital-based clinicians in delivering innovative technology solutions that dramatically improve patient safety. Welch holds bachelor’s degrees in both biomedical engineering and electrical engineering and is a Certified Clinical Engineer. He may be contacted at james.welch@soterawireless.com.