Sterile Compounding Tragedy: Symptom of a Broken System
November / December 2012
![]()
ISMP
Sterile Compounding Tragedy: Symptom of a Broken System
In the wake of the current multistate meningitis outbreak (www.cdc.gov/HAI/outbreaks/meningitis.html), ISMP wishes to express its deepest sympathy to all affected by the contamination of compounded products from a New England compounding pharmacy. This is certainly a tragedy of monumental proportions.
Past Drug Safety Issues
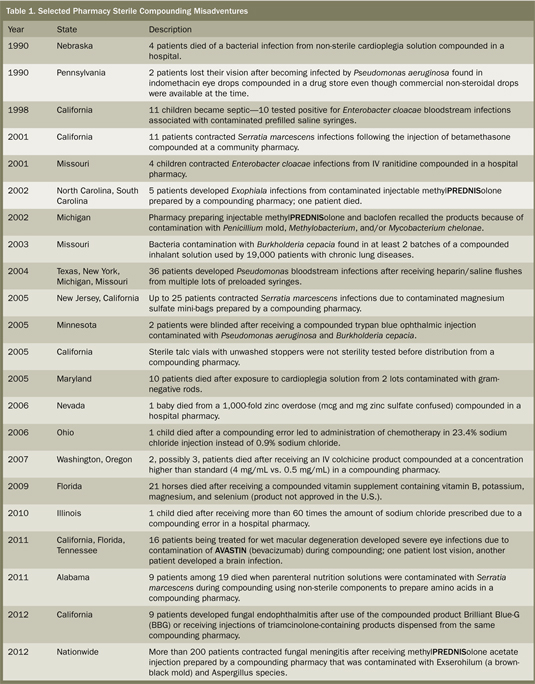
The scale of the meningitis outbreak could make it the worst among a series of fatal or harmful infections and overdoses linked to pharmacy compounding practices in the United States (Table 1), rivaling other key drug safety issues in the past that have led to substantial drug safety legislation. For example, in 1937, more than 100 patients died after the S. E. Massengill Company compounded an elixir of sulfanilamide using diethylene glycol (antifreeze), which it did not recognize as poisonous. At the time, safety testing of new products was not conducted. The tragedy was the driving force behind the 1938 Federal Food, Drug, and Cosmetic (FD&C) Act, which required drug safety testing for the first time. Then, exactly 50 years ago this past week (October 10, 1962), in response to severe birth defects associated with thalidomide, Congress passed the Kefauver-Harris Amendment to the FD&C Act, requiring drug manufacturers to provide proof of safety and effectiveness.
In the past two decades, there have been 200 adverse events involving 71 compounded products, many with devastating outcomes (McVeigh, 2012). Each case has prompted calls for federal oversight of compounding pharmacies that prepare sterile products, which are not subject to the same controls as drug manufacturers but whose regulation falls between the state board of pharmacy, the state department of health, and the U.S. Food and Drug Administration (FDA). While the current outbreak cannot be considered an outlier event, it should certainly be the very last event before enacting state and federal legislation to protect the public from preventable compounding errors and contamination.
Growth Brings Scrutiny
In the 1990s, FDA regulators began to scrutinize compounding pharmacies more closely as the number of facilities grew. According to David Kessler, then the commissioner of FDA, there was concern that the drug compounding pharmacies would spawn a “shadow industry” of drug distributors that “could result in serious adverse effects, including death”—a rather prophetic statement, given the current outbreak. The compounding pharmacy in the current case was not registered with FDA and is being investigated for crossing the line into full-scale manufacturing by taking bulk orders for methylPREDNISolone without proof of individual prescriptions and shipping large batches of drugs nationwide, a practice FDA had previously warned the company about in regards to other drugs. (Other compounded products prepared by the New England pharmacy, including triamcinolone injection and cardioplegia solutions, are also suspected to be contaminated.) While it is difficult to understand how authorities could have overlooked the transition to full-scale manufacturing, the pharmacy has been accused of misleading regulators.
The 2008 revision of the US Pharmacopeia (USP) Chapter that left many facilities unable to meet the published standards for sterile compounding and the escalation in drug shortages have led to a steady increase in sterile compounding pharmacy services. A 2011 survey showed that 66% of hospital pharmacies outsource some portion of their sterile compounding (Pharmacy Purchasing & Products, 2011). In some cases, pharmacists have purchased compounded products without full realization of the risks. An analysis of recent harmful cases of contaminated products from compounding pharmacies revealed breaches of USP , unsafe staff behaviors, untrained and unskilled personnel, improper use of equipment, extended-beyond-use dating outside of manufacturer labeling without sufficient testing, and/or a lack of basic compounding skills involved in almost all cases (Kastango E. Personal communication to Michael R. Cohen. October 14, 2012). Outsourcing is also used as a cost-savings measure. Purchase of commercially available products from drug manufacturers is optimal because they are FDA-approved and known to already meet good manufacturing practices for quality. We now need similar regulations that will ensure the same level of safety with pharmacy-compounded products.
Prior Legislative Efforts
In the wake of the recent outbreak, several members of Congress have promised to introduce legislation giving FDA greater authority over compounding pharmacies.?But this is not the first time Congress has pushed for such authority—legislation in 1997, 2003, and 2007 was either overturned on appeal or defeated via industry lobbying. It isn’t known whether tighter regulations would have stopped the latest outbreak, but the 2007 bill proposed restricting distribution of compounded drugs across state lines, which could have prevented contaminated products from reaching facilities outside of Massachusetts.
Need For Future Oversight
As we move forward and learn from the most recent outbreak, we call upon Congress to work with key stakeholders to address the need for additional laws on the federal level to fill in regulatory gaps. FDA oversight and regulations must be equal to what is required of pharmaceutical companies when compounding pharmacies dispense in mass quantities without individual patient prescriptions, manufacture sterile products from non-sterile active ingredients, or distribute across state lines. These pharmacies must be registered with FDA and be subjected to regular inspections. Congress must clearly identify FDA as the regulatory authority of these pharmacies. For compounding pharmacies that dispense sterile products only upon receipt of an individual patient’s prescription, FDA must support the state boards of pharmacy in their oversight of these pharmacies and standardize the expectations regarding the state boards’ role in ensuring compounding safety. We further recommend that FDA establish nomenclature to differentiate these two types of compounding pharmacies so that healthcare providers employing their services understand the structure, requirements, and regulatory oversight of the pharmacies with which they are doing business. We also encourage FDA to move forward with plans to publish guidances on Good Pharmacy Compounding Practices for Sterile Drug Products and Outsourcer Pharmacy Operations Compliance Policy Guide (www.ismp.org/sc?id=115).
We call upon Congress and appropriate state authorities to evaluate the construct of state boards of pharmacy and their role in keeping patients safe. There are five state boards that do not even mention the need for compounding pharmacies to adhere to USP . In other states, the standard isn’t mentioned but aspects of it are incorporated into a state regulation. State boards of pharmacy must require compounding pharmacies to comply with all aspects of USP and survey the pharmacies regularly for compliance. To do this, pharmacy boards must be provided with additional resources to adequately train and deploy inspectors to assess compliance. Without additional resources, the assignment of responsibility will not result in improved oversight. There should also be regulatory oversight of non-sterile compounding to ensure compliance with USP .
We call upon healthcare providers to use commercially available, ready-to-use, FDA-approved products from pharmaceutical manufacturers as often as possible. When products are not commercially available, providers, including physicians, must carefully assess and select a compounding pharmacy for medically necessary medications. Guidelines for outsourcing or selecting such a pharmacy for sterile compounding services are available from the American Society of Health-System Pharmacists (ASHP) (www.ismp.org/sc?id=111). The ASHP Foundation also provides a contractor assessment tool for outsourcing preparation of sterile products (www.ismp.org/sc?id=112).
We call upon The Joint Commission to establish a standard requiring pharmacy evaluation and monitoring of the quality of any supplier of compounded products and to consistently survey compliance with the USP standard as it relates to the type of compounded products being prepared in accredited facilities in all states.
We call upon all pharmacists and pharmacy technicians who compound sterile preparations, regardless of where they work, to know and comply with USP . Compounding errors can happen in any setting, and the impact of a single misadventure is just as devastating to the single patient it effects as with each of the victims affected by the latest national outbreak. We also recommend establishing an internal quality surveillance and review team within your organization to regularly test compounded preparations and monitor the environment, compounding equipment, and personnel for compliance with key aspects of USP. An April 2011 supplement to Pharmacy Purchasing & Products on the state of pharmacy compounding is an excellent resource to help guide this type of surveillance and improvement process. Any problems uncovered during surveillance require immediate stoppage of compounding, investigation, corrective action, and revalidation before resumption of activities. All pharmacy staff members have a moral and legal obligation to compound preparations using the least risky processes while adhering to the highest standards possible. Even the slightest misstep could result in a disaster such as the tragedy before us.
Lessons Learned
We don’t have all the details about this recent outbreak, but a crucially important lesson we can take away from this tragedy is that we all need to make improvements based on the outcome of this investigation. Unfortunately, there are too many individuals in healthcare who feel that, if it hasn’t happened to them, the adverse experiences of others do not apply. If investigation into this event uncovers some aspect (e.g., frequency of testing staff samples and environmental samples) of compounding that was overlooked in either the USP standard or in staff practices, then we must learn from it, incorporate necessary changes, and provide leadership and oversight to assure our patients are kept safe. We must find a way to be able to trust that the providers of these compounded products that are not commercially available are producing safe and effective products when needed.
ISMP hopes to contribute to the national dialogue and legislative action to improve oversight of compounding pharmacies to maximize patient safety. Again, we extend our deepest sympathy to those affected by the use of contaminated products and to the family and friends of those who died. As an organization that cares deeply about patient safety, we will not let the latest round of tragedies go unchallenged and the latest round of victims to be forgotten. But we are also an organization that recognizes the destructive forces of hindsight bias, and we care deeply about the second victims of this tragedy—the healthcare providers and others who were involved in these adverse patient outcomes in some way. While personal accountability for the quality of ones’ behavioral choices is imperative to safety, we recognize that the staff involved in the latest outbreak could also be victims of a system that allows unsafe practices to flourish in a highly unregulated industry. While we have read comments in the media that suggest a commitment to ensuring that all the responsible staff at the New England pharmacy are identified and punished, we cannot support the outcome bias that accompanies such a proclamation. We remind our colleagues that the latest event is a symptom of a broken system on many levels and that there are a multitude of victims that deserve our support and compassion. Our hearts and prayers go out to all the victims and their families.
This column was prepared by the Institute for Safe Medication Practices (ISMP), an independent, nonprofit charitable organization dedicated entirely to medication error prevention and safe medication use. Any reports described in this column were received through the ISMP Medication Errors Reporting Program. Errors, close calls, or hazardous conditions may be reported online at www.ismp.org or by calling 800-FAIL-SAFE (800-324-5723). ISMP is a federally certified patient safety organization (PSO), providing legal protection and confidentiality for patient safety data and error reports it receives. Visit www.ismp.org for more information on ISMP’s medication safety newsletters and other risk reduction tools.This article appeared originally in the October 18, 2012 issue of the ISMP Medication Safety Alert!
Acknowledgment
ISMP thanks Eric S. Kastango, MBA, RPh, FASHP, CEO of ClinicalIQ, for his contribution to the table and for serving as an expert reviewer for the related article (www.clinicaliq.com).