Crossing the Medication Safety Chasm
New technology identifies, measures, and documents manual intravenous bolus injections in real time
By James H. Philip, ME(E), MD, CCE; Mark Mitchell, MD, MSEE; and Celine Peters, RN, MN
Anesthesiologists have long played a role in advancing medication safety. More than 30 years ago, the Anesthesia Patient Safety Foundation (APSF) was launched as an independent organization with the vision that “no patient shall be harmed by anesthesia” (Stoelting, n.d.). Recognizing the importance of multidisciplinary collaboration, the APSF includes anesthesiologists, nurse anesthetists, nurses, manufacturers of equipment and drugs, regulators, risk managers, attorneys, insurers, and engineers.
Over the years, APSF initiatives to improve anesthesia-related medication safety have helped achieve a 10- to 20-fold reduction in mortality and catastrophic morbidity for healthy patients undergoing routine anesthetics. This, in turn, has led to the dramatically reduced insurance risk-relativity rating of anesthesiologists compared with other specialties (Stoelting, n.d.). But the work is not done.
In January 2016, Anesthesiology published the largest observational study of anesthesia-related medication errors to date. In this prospective clinical study by Nanji, Patel, Shaikh, Seger, and Bates, direct observation by fully trained, experienced anesthesia providers (AP) found a medication error rate substantially higher than rates found through self-reporting or surveys (Orser, Chen, & Yee, 2001). Nanji et al. also identified several technology- and process-based improvement strategies.
In keeping with anesthesiology’s history of improving medication safety, APs have been working with a leading medical technology company for several years to help develop a new intravenous (IV) injectable medication safety system. The resulting first-of-its-kind device was cleared for clinical use by the FDA in December 2014 (Figures 1–2) and is commercially available today.
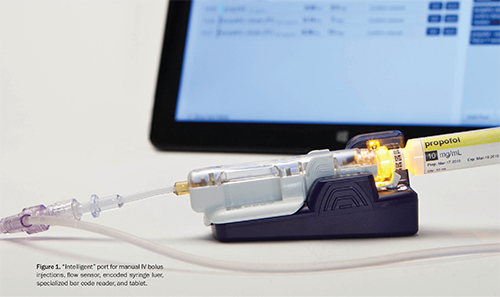
This article presents an overview of the need for improved medication safety for manual IV bolus injections, recommendations and strategies for improvement, the new FDA-cleared technology, and proposed advantages of this new technology’s use in the perioperative setting.

Need for improved perioperative medication safety
APs in the operating room (OR) are the only medical personnel who autonomously prescribe, secure, prepare, administer, and document medications (Eichhorn, 2010)—a process that can take more than 40 steps per medication administration (Martin, 2008). These steps usually are performed within a very short interval, often in a distracting environment, and typically without standardized protocols (Eichhorn, 2010).
Medications delivered during anesthesia are most often administered by IV injections. Of these, 89% are high-alert meds that can cause significant patient harm when used in error (ISMP, 2014). Hicks, Becker, and Cousins (2007) reported that harm was three times higher in perioperative patients than non-perioperative patients.
In a 2002 study by Flynn, Barker, Pepper, Bates, and Mikeal, “Observers detected 300 of 457 pharmacist-confirmed errors made on 2,556 doses (11.7% error rate) compared with 17 errors detected by chart reviewers (0.7% error rate), and 1 error detected by incident report review (0.04% error rate)” (p. 436).
Nanji et al. (2016) found that “approximately 1 in 20 perioperative medication administrations included a medication error and/or adverse drug event (ADE). More than one third of these errors led to observed patient harm, and the remaining two thirds had the potential for harm” (pp. 32-33). Of the 193 events detected, 153 (79.3%) were deemed preventable.
In one recent study, incorrect doses, incorrect medications (look-alike vial, ampoule, or syringe), and missed doses accounted for more than 80% of OR medication errors (Cooper, Digiovanni, Schultz, Taylor, & Nossaman, 2012). The study also revealed that inexperience, haste,distraction, and misread labels were common causes. All of these are human errors that are amenable to prevention. However, perioperative medication administration today often bypasses standard safety checks such as electronic physician order entry with decision support, pharmacy approval of specific drugs before administration, and final safety checks at the time of administration (Nanji et al., 2016). The high-stress, time-sensitive nature of OR care may also contribute to higher rates of high-severity medication errors in this setting. In Nanji et al., most practitioners did not document medications until some time after they were administered.
Accurate documentation is another area of concern. Inaccurate documentation can undermine clinical decision-making, understanding of the physiological effects of medications delivered, and charge capture. Accurately recording the drug, exact dose, and time of administration can be difficult for a single provider using a manual paper record or computer system, especially since many electronic systems record medication administration only in 1-minute or 5-minute increments. Avidan, Dolan, Weissman, Cohen, and Levin (2014) found documentation inaccuracies in 15% of cases when using a manual anesthesia information management system. The task of documentation can also take the AP’s attention away from the patient.
Strategies to minimize medication errors
Education alone does not suffice to reduce medication errors; the adoption of new technologies should also be considered (Orser, U, & Cohen, 2016). Specific solutions exist that may decrease the incidence of perioperative medication errors.
- Anderson, Jay, Anderson, and Hunt (2002) demonstrated a 26.3% reduction in medication errors using integrated information technology in simulation. This reduction may be attributed to more legible medication entries, immediate availability of reports to the next care area, and decision support reminders.
- Poon et al. (2010) found that introduction of a barcoding medication administration system (BCMA) in a nursing unit reduced errors such as administering the same medication twice, medications that have expired or been discontinued, or no medication order found.
- A study by Nolen and Rodes (2008) demonstrated that using a BCMA system in the OR increased the quantity of anesthetic drugs documented per case by 21.7% and drug revenue capture per case by 18.8%.
- Merry et al. (2011) demonstrated a 21% reduction of perioperative medication errors using additional safety components such as on-screen visual and auditory warnings, an automated anesthesia record, and a paper printout at the end of anesthesia for final review.
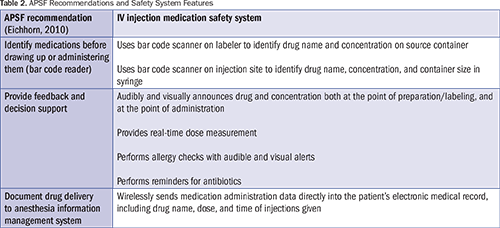
The APSF has recommended that every anesthetizing location should have a mechanism to identify medications before drawing up or administering them (e.g., bar code reader) and a mechanism to provide feedback, decision support, and documentation (automated information system) (Eichhorn, 2010). In 2015, the Institute for Safe Medication Practices (ISMP) recommended appropriate labeling of all clinician-prepared syringes for IV push medications or solutions, and the use of bar code scanning or similar technology immediately prior to the administration of IV push medications to confirm patient identification and the correct medication (ISMP, 2015). Nanji et al. (2016) identified both process- and technology-based interventions to minimize perioperative medication errors and adverse drug events. Examples of technology interventions include barcode–assisted syringe labeling systems, anesthesia documentation systems, specific drug decision support, and alerts.

APs must be able to ensure that the desired drug and concentration is about to be administered. And, if there is reason that a particular drug should not be given or that further considerations are warranted (drug allergy, abnormal sensitivity, cross-reactivity, or incorrect sequence), this information must be conveyed to the AP before administration. Patient safety organizations such as APSF have stated that drug identification is needed. Further, communicating medication administration data in real time to automated recordkeeping systems would ensure accurate, timely documentation.
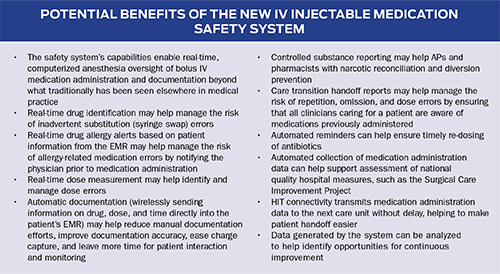
All these needs would be met by an IV injectable medication safety system that, upon connection of the syringe to the injection port, immediately identifies the medication name and concentration; alerts the AP to considerations such as a patient-specific allergy to the medication; measures the exact drug, dose, and time of administration; and records that information in the patient’s medical record. Such technology could help streamline the AP workflow, provide repeated checks, and improve the timeliness and accuracy of medication administration documentation. It would also lay the groundwork for further research into dose and time response to medications used during anesthesia.
New technology to help manage manual IV bolus injections
The new IV injectable medication safety system (BD IntelliportTM Medication Management System,a-g BD, Franklin Lakes, NJ) is the first system to integrate advanced software, wireless connectivity, label-encoded syringes, touch-screen tablet technology, a unique IV injection site with a specialized bar code reader for drug identification, and an ultrasound flow sensor to measure volume administered (Table 1). By integrating these components, the system provides real-time drug identification, dose measurement and allergy detection at the point of injection, and wireless transmission of medication administration data directly into the patient’s electronic medical record (EMR) (Figure 2).
The system can be used with a paper anesthesia record and, if integrated with hospital information technology (HIT), can send medication administration data directly to the EMR for the AP to review and document. The information is immediately available and can follow the patient throughout the perioperative stay. The system’s fluid path is sterile, single-patient use, safe for all injectable medications, and biocompatible for patient contact. As shown in Table 2, system features meet APSF recommendations (Eichhorn, 2010).
Preparation
The labeler generates specially encoded syringes for use with the safety system. When the source container is scanned with the built-in bar code reader (Figure 3), the labeler provides audible and visual announcements of drug name and concentration, which may help manage vial/ampoule swap errors. The labeler then generates and automatically applies an encoded label with that information to the luer collar of the syringe (Figure 4).
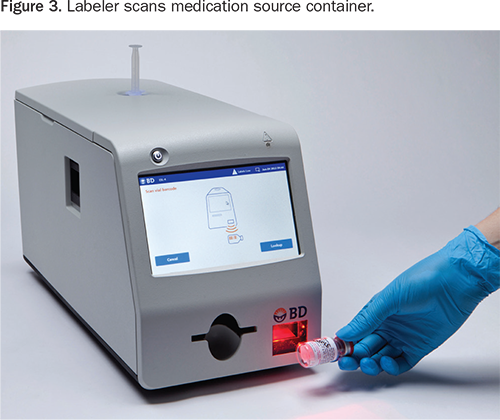
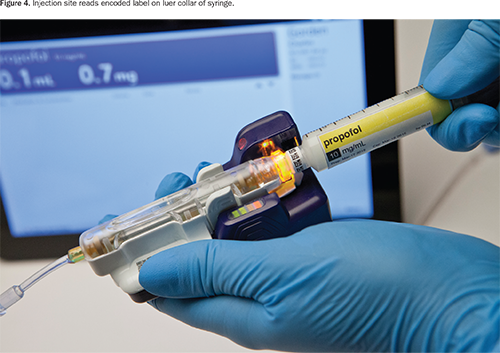
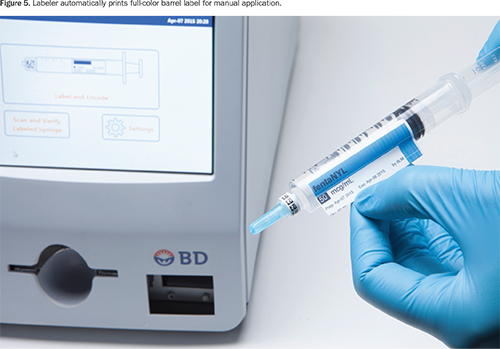
The labeler simultaneously prints a full-color barrel label that contains the medication name, concentration, expiration date, and hospital-customized information based on Joint Commission, ASTM, or American Society of Anesthesiologists labeling guidelines (Figure 5). The clinician applies the barrel label manually.
The patient’s information, including a summary of any allergies (medication name, severity, and reaction, if known), is imported by the safety system from the EMR and displayed on the tablet setup screen. The hospital-specific drug formulary is also imported and displayed. The injection site’s unique serial number is wirelessly transmitted to the tablet by the sensor and also displayed on the screen. The tablet screen will display the serial number of the sensor, and the clinician will match the serial number displayed on the screen with that on the patient’s sensor.
Administration
During syringe luer-lock connection to the injection port, the system reads and wirelessly transmits the bar-coded information on the syringe luer to the tablet, which visually and audibly announces the syringe medication and concentration in real time. The system then checks for patient-specific allergies and provides an alert immediately upon syringe connection if any are detected. The system does not prevent the medication from being delivered if an allergy alert is displayed.
During injection, the ultrasound sensor measures flow, and the system indicates flow-speed adequacy (≥ 10mL/min) through lights located on the port and visual alerts on the tablet display. The software calculates the dose administered as fluid volume times the concentration of the drug and displays the information in real time on the tablet screen. (Video demonstration is available at www.bd.com/intelliport.) The medication administration data (name, concentration, dose, and time the drug was given) is recorded automatically and displayed on the tablet for the AP’s use during administration.
Documentation
Medication administration data are saved on the tablet and sent wirelessly through the HIT network to be automatically entered into the patient’s EMR without additional clinician input. The system can also be configured for AP review prior to or after documentation. The stored data can be used to create quality reports that provide a detailed log of activities during a case; information on the use of antibiotics, reversal agents, beta blockers, and controlled substances; near-miss events; the number of allergy alerts; medication charge capture; and other information logged by the system. Reports can be generated for a specific clinician (if the log-in feature has been enabled), time period, and case(s). Reports can also identify specific cases for review and additional follow-up.
Next steps
As noted above, the IV injectable medication safety system was cleared by the FDA for clinical use in December 2014 and is commercially available today. Clinician feedback from recent simulation studies is now being used to design further clinical study research. A prospective, observational, multi-center study designed to capture data to demonstrate the system’s potential clinical and workflow impact is planned for 2016.
Conclusion
While process improvements are needed, the new technology holds the promise of helping to streamline workflow, improve medication safety, and provide more accurate and timely documentation. It also lays the foundation for further research and improvements in OR clinical and financial performance. As with earlier initiatives, the new IV injectable medication safety system is the result of APs and technology experts working together to fulfill the APSF vision that “no patient shall be harmed by anesthesia.”
Footnotes:
- BD Intelliport™ Medication Management System, Becton, Dickinson and Company, Franklin Lakes, NJ
- BD Intelliport™ Injection Site, Becton, Dickinson and Company, Franklin Lakes, NJ
- BD Intelliport™ Sensor, Becton, Dickinson and Company, Franklin Lakes, NJ
- BD Intelliport™ Base, Becton, Dickinson and Company, Franklin Lakes, NJ
- BD Intelliport™ Tablet, Becton, Dickinson and Company, Franklin Lakes, NJ
- BD Intelliport™ Gateway, Becton, Dickinson and Company, Franklin Lakes, NJ
- BD Intelliport™ Syringe Labeler, Becton, Dickinson and Company, Franklin Lakes, NJ
Further information is available at www.bd.com/intelliport.
BD, BD Logo, and BD Intelliport are trademarks of Becton, Dickinson and Company. © 2015 BD BD-0744
James Philip is an anesthesiologist at Brigham and Women’s Hospital and professor of anesthesia at Harvard Medical School. He holds bachelor’s and master’s degrees in electrical engineering from Cornell and a medical degree from SUNY Upstate, and is a Certified Clinical Engineer. He worked as a design engineer for Hewlett Packard Medical throughout his engineering and medical education. He is a member of the Harvard Anesthesia Risk Management Committee and was co-author of the Harvard Anesthesia Monitoring Standard of 1984. Philip developed the Gas Man® educational computer, which simulates inhaled anesthetic gas delivery, distributed by Med Man Simulations, a nonprofit charitable organization (www.gasmanweb.com). He also created commercial medical products, including the Edwards Vigilance® Continuous Cardiac Output Monitor, the Perkin-Elmer Lifewatch™ CO2 Monitor, the Baxter InfusOR™ IV Pump for Bolus Plus Infusion, the multiple-manufacturer Pressure Infuser for IV fluid resuscitation, and the BD Signature™ Pump to monitor hydraulic resistance and detect catheter infiltration.
Mark Mitchell is an anesthesiologist. He was the anesthesia service chief at VAMC San Diego from 1996 to 2014 and was with the UCSD Department of Anesthesiology from 1977 through his retirement in 2014. Mitchell holds bachelor’s and master’s degrees in electrical engineering from MIT. He attended UCSD Medical School and completed his anesthesia residency and bioengineering fellowship at Massachusetts General Hospital. Mitchell’s subspecialty interests and experience include physiological monitoring and automated anesthesia recordkeeping, cardiac anesthesia, pediatric anesthesia, and perioperative transesophageal echocardiography. He served a fellowship in echocardiography and cardiac anesthesia at the Ditzik Hospital, Erasmus University Thoraxcentrum in Rotterdam, The Netherlands.
Celine Peters is a clinical nurse specialist at Becton Dickinson for the Intelliport project. She holds a master’s degree in nursing from UCLA. Peters has over 20 years’ experience, including 12 years as a director of clinical research and clinical support for several pharmaceutical and medical device companies. Most recently she worked at Sonosite (formerly CardioDynamics), where she was responsible for clinical research activities and multi-center trials, plus education and staff training; served as clinical liaison to the sales team; and conducted numerous formal presentations across the United States. Additionally, she is a clinical nurse specialist and has held administrative hospital positions focused on the outcomes of clinical care, quality management, and patient safety. She is published in the area of outcomes and the influence of hemodynamics on disease states. She has been an associate professor of nursing at UCLA, and a past board member of the American Heart Association, San Diego. Peters may be contacted at celine_peters@bd.com.
References
Anderson, J. G., Jay, S. J., Anderson, M. M., & Hunt, T. J. (2002). Evaluating the capability of information technology to prevent adverse drug events: A computer simulation approach. Journal of the American Medical Informatics Association, 9(5), 479–490.
Avidan, A., Dolan, K., Weissman, C., Cohen, M. J., & Levin, P. D. (2014). Accuracy of manual entry of drug administration data into an anesthesia information management system. Canadian Journal of Anesthesia, 61(11), 979–985.
Cooper, L., Digiovanni, N., Schultz, L., Taylor, A. M., & Nossaman, B. (2012). Influences observed on incidence and reporting of medication errors in anesthesia. Canadian Journal of Anesthesia, 59, 562–570.
Eichhorn, J. H. (2010). Consensus group defines challenges and opportunities for improved practice. APSF Newsletter, 25(1), 7.
Flynn, E. A., Barker, K. N., Pepper, G. A., Bates, D. W., & Mikeal, R. L. (2002). Comparison of methods for detecting medication errors in 36 hospitals and skilled-nursing facilities. American Journal of Health-System Pharmacy, 59, 436–446.
Hicks, R. W., Becker, S. C., & Cousins, D. D. (2007). MedMARx data report: A chart book of medication error findings from the perioperative settings from 1998–2005. Rockville, MD: United States Pharmacopeia.
Institute for Safe Medication Practices (ISMP). (2014). ISMP list of high alert medications in acute care settings. Retrieved from https://www.ismp.org/tools/highalertmedications.pdf
Institute for Safe Medication Practices (ISMP). (2015). Safe Practice Guidelines for Adult IV Push Medications. Retrieved from http://www.ismp.org/Tools/guidelines/ivsummitpush/ivpushmedguidelines.pdf
Martin, D. E. (2008). Medication errors persist: Summit addresses intravenous safety. APSF Newsletter, 23(3), 37–39.
Merry, A. F., Webster, C. S., Hannam, J., Mitchell, S. J., Henderson, R., Reid, P., … Short, T. G. (2011). Multimodal system designed to reduce errors in recording and administration of drugs in anaesthesia: Prospective randomised clinical evaluation. BMJ, 343, d5543.
Nanji, K. C., Patel, A., Shaikh, S., Seger, D. L., & Bates, D. W. (2016). Evaluation of perioperative medication errors and adverse drug events. Anesthesiology, 124(1), 25-34. doi: 10.1097/ALN.0000000000000904
Nolen, A. L., & Rodes, W. D. (2008). Bar-code medication administration system for anesthetics: Effects on documentation and billing. American Journal of Health-System Pharmacy, 65(7), 655–659.
Orser, B. A., Chen, R. J., & Yee, D. A. (2001). Medication errors in anesthetic practice: A survey of 687 practitioners. Canadian Journal of Anesthesia, 48, 139–146.
Orser, B. A., U, D., & Cohen, M. R. (2016, January). Perioperative medication errors: Building safer systems [Editorial views]. Anesthesiology, 124, 1–3.Retrieved from http://anesthesiology.pubs.asahq.org/article.aspx?articleid=2466533
Poon, E., Keohane, C., Yoon, C., Ditmore, M., Bane, A., Levtzion-Korach, O., … Gandhi, T. (2010). Effect of bar-code technology on the safety of medication administration. New England Journal of Medicine, 362, 1698–1707.
Stoelting, R. K. (n.d.). APSF Foundation history. Retrieved from http://www.apsf.org/about_history.php
