Interdisciplinary Leadership Team Responds to Escalating Drug Shortages
By Neil A. Gilchrist, PharmD, BCPS; Fran Bassett, RPh, BSPharm; Roland Bercume, MS, RPh; Denis Brown, RPh, FASCP; Robert Klugman, MD
Medication shortages have steadily increased over the past decade due to a number of factors affecting drug supply. Perhaps the first and worst hit has been the hematology/oncology area, which has been dealing with shortages and the need to redesign chemotherapy protocols for a number of years. Within the past three years we have seen an increasing trend of drug shortages that have occurred and continue with no resolution or intermittent supplies returning to the market.
In June 2012, the U.S. House of Representatives Committee on Oversight and Government Reform published a staff report on the FDA’s contribution to the drug shortage crisis. One of the major findings of the committee included the FDA regulatory activity that shut down 30% of the total U.S. manufacturing capacity at four of the largest producers of generic injectable medications. One major contributing factor was the change in oversight of the FDA by newly appointed FDA Commissioner Margaret Hamburg in June 2009. As commissioner, she set a priority to increase enforcement activity and ensure compliance of regulations by manufacturers. Other contributing factors to the current drug shortage crisis include a concentrated manufacturing market, the use of group purchasing organizations (GPOs) driving down drug pricing, and the development of the Medicare Modernization Act (MMA), affecting the reimbursement paid by Medicare for many generic injectable medications.
The impact of drug shortages has not been fully investigated, however, there is a significant impact on patient care, finances, and operational workflow, which has been widespread. This has mainly affected the hospital, long-term care, and specialty clinic settings as the majority of new drug shortages are generic intravenous injectable drugs. More recently, critical life-saving medications and electrolytes have been constantly in short supply, requiring the full support of healthcare leadership in order to facilitate changes in practice, as well as advocate for additional resources both for pharmacy staff and more costly substitute drugs.
Metzger and colleagues (2012) presented the findings of clinical impact related to a drug shortage of mechlorethamine, a nitrogen mustard used as part of a 12-week chemotherapy regimen for pediatric Hodgkin’s lymphoma known as the Stanford V regimen. The shortage forced the group to use an alternative regimen swapping the mechlorethamine for cyclophosphamide. In a retrospective comparison, the 40 patients that received the modified Stanford V regimen showed significantly less effectiveness, with a reduced 2-year event-free survival of 75% compared to 88% (P=0.01) in the original Stanford V regimen with mechlorethamine.
In the non-oncology arena, drug shortages often result in substitution of another equivalent agent or reduction in utilization. For example, during a shortage of injectable prochlorperazine, a medication used for the treatment of nausea and emesis, alternative drugs such as ondansetron or droperidol injection could be substituted with equivalent efficacy. As the majority of healthcare organizations increased purchases of the alternative anti-emetics, a national shortage of all injectable drugs to treat nausea and emesis resulted.
While information is shared about shortages across organizations, every organization is handling its approach to these shortages individually. Our organization has followed published recommendations in order to establish short- and long-term strategies to effectively treat our patients (Fox et al., 2009). Figure 1 describes our standard procedure for drug shortage assessment and management. The following sections provide a description on the scope and membership that contribute to our drug shortage committees.
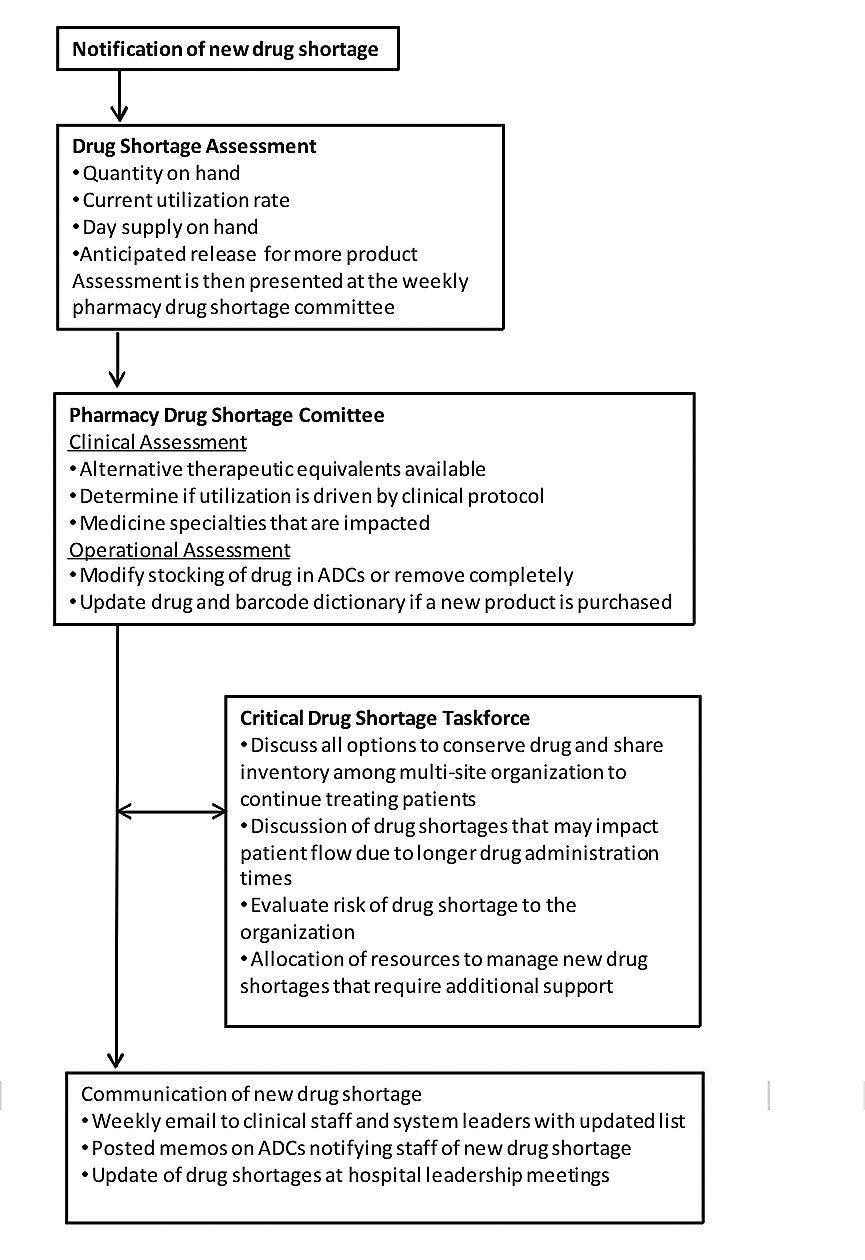
Figure 1. Procedure for Drug Shortage Assessment and Management
Pharmacy Drug Shortage Committee
In 2009, the department of pharmacy at UMass Memorial Healthcare, a five-hospital system located in central Massachusetts, developed a committee to meet weekly and ad-hoc to discuss current drug shortages and new shortages affecting drug supply. This committee includes directors of pharmacy, pharmacy buyers, clinical manager, operation managers, and clinical pharmacists. The committee reviews each current and new drug shortage by a full risk assessment to include evaluation of stock on hand, current utilization rates, and estimated time to depletion of supply. In the majority of shortages, drug utilization can be reduced either by increasing the restriction or criteria for therapy with the drug in shortage or by using an alternative medication that is considered therapeutically equivalent.
Within the past year, the severity of these drug shortages has become even more critical, impacting life-sustaining, life-saving medications and drugs that can be substituted with an equivalent agent but at the expense of potential increased adverse effects. The FDA has responded to some of these shortages more recently by working with manufacturers to extend stability dating beyond the printed expiry on the drug product label, allowing product into the market with additional filtration requirements and importation of drug product from outside of the United States. The fast moving and rapid escalation of critical drug shortages forced our organization to look at how we handle these shortages.
Critical Drug Shortage Taskforce
In October 2012, an outbreak of fungal meningitis occurred in many states in the U.S. due to contaminated epidural spinal injections linked back to a compounding pharmacy, New England Compounding Company (NECC). In this outbreak, more than 700 patients were infected with fungal meningitis, and 64 people died. This event could not have happened at a worse time for drug shortages, as NECC’s sister company, Ameridose, was a major supplier for many hospitals of drugs in short supply from the manufacturer. During a drug shortage, compounding pharmacies, such as Ameridose, serve the role of sterile admixing large bulk vials of medications into smaller, ready-to-use form. This occurs when individual hospitals can either not purchase any more of the drug themselves or do not have the staff to absorb the additional workload of admixing sterile products previously available from a manufactured source. Ameridose was shut down by the FDA as part of the investigation of the parent company NECC.
In response to the NECC scandal, UMass Memorial Healthcare formed an ad hoc committee to address the impact of both the use of NECC and Ameridose products throughout our organization, as well as its impact on an already growing series of critical drug shortages occurring across the U.S. and impacting our ability to provide care. We engaged representatives from management, quality, pharmacy, nursing, infectious diseases, critical care, hematology-oncology, legal, risk management, and other clinicians in this effort.
As the NECC issues resolved, the focus of the committee shifted to the drug shortage crisis. Our aim was to stay abreast of the rapidly changing drug shortage environment, oversee interdisciplinary efforts to address specific shortages, support the pharmacy in its efforts to manage the changes in agents, dose or vehicle changes, alternative agent options, and the associated changes in clinical practices required to adopt these changes. In addition, it became increasingly apparent that we needed to “skate ahead of the puck” in terms of anticipating likely shortages and make changes in anticipation of the impact.
The critical drug shortage taskforce accomplished three goals. First, the pharmacy, in conjunction with the department of quality, developed a real-time dashboard of agents in short or no supply, including anticipated resolution of availability, when known, and an intuitive scoring system designed to assist clinicians in addressing necessary changes in practice (Figure 2, page 35). Second, education of leadership and clinicians in the factors leading to these shortages, the procurement process, and the types of actions required to ensure safe patient care. Third, the system streamlined the response to a specific critical shortage, allowing for a rapid, effective response to the shortage. The committee meets monthly and on an ad hoc basis, depending on the need. The chief medical officer (CMO) and chief quality officer (CQO) work with the pharmacy between meetings as issues arise to facilitate the engagement of the clinicians and to help with difficult decisions.
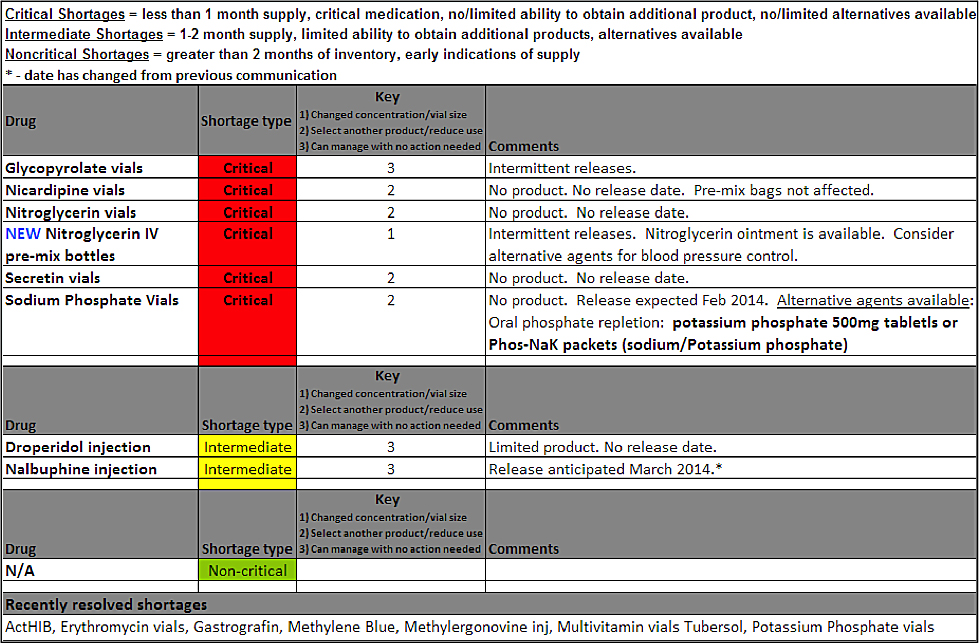
Figure 2. Drug Shortage Communication Format
The types of interventions fall into 3 categories:
Substitution of the same agent in a different concentration or vehicle. This requires education and appropriate labeling of the substituted product, and in some cases requires the pharmacy to repackage larger volumes of the agent into unit doses. Indirect impacts included subsequent shortages of the alternate drug therapies and significant increases in cost, time, and effort in preparation.
Substitution of a like agent where safe. This requires close collaboration with the clinicians using this agent and an understanding of the disease state being treated. Indirect impacts included subsequent shortages of the alternate drug therapies, and significant increases in cost, time, and effort in preparation.
Rationing of an agent. Working with clinicians, we either sharply defined the true indications for using an agent, prioritized the disease states requiring this agent or, in some cases, cessation of the type of treatment requiring these agents by switching to an alternative therapy or cancelling/redirecting patients. This has been the highest risk area, particularly where agents are required in life threatening situation and in the pediatric population.
Below are three such examples of critical drug shortages that have been escalated to our critical drug shortage task force due to the nature of the medication and broad use.
Case 1. Propofol Shortage
Over the past 2 years, propofol has been intermittent in supply to healthcare systems. This agent is commonly used for procedural sedation along with continuous sedation of mechanically ventilated patients impacting a broad range of patients. During the course of this shortage, our organization was faced with a significant supply issue and estimated that we had less than a five-day supply on hand at one point.
Due to the critical nature of this drug, the shortage was escalated to the critical drug shortage taskforce.
The taskforce enlisted the help of our critical care leadership and anesthesia department to immediately reduce use. Alternative sedative agents were discussed along with any supply issues expected for these medications. All patients that were on continuous infusions of propofol were switched to an alternative sedative infusion unless frequent neurologic checks were needed. Anesthesiologists used alternative sedative drugs in the OR setting as well. Our group drafted a system wide memo to all prescribers educating them on the propofol shortage and need to conserve remaining supplies.
Patients in the intensive care units were switched to alternative sedation agents, reducing use of propofol by more than 80% in this population along. Additional conservation efforts included a change in procedure allowing the OR setting to continue use of propofol for anesthesia purposes, using smaller volumes on a syringe pump.
Case 2. Intravenous Phospholipids
In January 2013, a new shortage of intravenous lipid emulsions developed, a major component of intravenous total parenteral nutrition (TPN). Our weekly allocation from the wholesaler did not match our current utilization with a projected six-week supply on hand. Unsure of when the shortage would resolve, we were forced to decide on a rationing plan and escalate the shortage to the critical drug shortage taskforce.
In order to provide this medication to all of our parenteral nutrition patients that span all age categories and services, we invited the medical director for our Nutrition Advisory Committee to the critical drug shortage taskforce and requested a change in administration practice for IV lipid emulsion for our adult population. This change would include both acute and critical care patients across our system. With the support of the Nutrition Advisory Committee and the division chiefs of all medicine and surgery departments as well as senior physician administrative leadership, we were able to change from daily administration of IV lipid emulsion to lipid administration three times weekly in our adult population. The committee did not change the neonatal or pediatric populations’ lipid administration schedule due to their critical nutritional needs.
This practice change reduced our lipid utilization by more than 40% with no impact on patient outcomes. Department chiefs, nutrition support dieticians, and clinical pharmacists were all available to assist with orders and questions after this process change to three times weekly administration of IV lipids. Any patient that needed additional lipids was evaluated by the nutrition support team and additional lipids were given if deemed necessary for that patient’s clinical picture. No patient experienced fatty acid deficiency or any untoward effect by receiving IV lipid emulsion only three times weekly.
Case 3. Intravenous Phosphate
In early December 2012, the two manufacturers of injectable sodium phosphate reported manufacturing delays resulting in decreased availability of product on the market. At the same time, injectable potassium phosphate, which was already in short supply, also experienced manufacturing delays by the same manufacturers. This resulted in a significant decrease in the availability of injectable phosphate products over the next six months, forcing the escalation of this shortage outside of the pharmacy drug shortage committee to our critical drug shortage taskforce.
The taskforce discussed current utilization compared to on-hand inventory. By late January 2013, our supply of injectable potassium phosphate was less than one week away from being completely depleted. At the same time, the Critical Drug Shortage Taskforce needed to conserve remaining injectable sodium phosphate. Table 1 describes the actions taken to reserve injectable phosphate products. Within a few weeks these high-level decisions were moved through the appropriate committees and approved for change in practice.
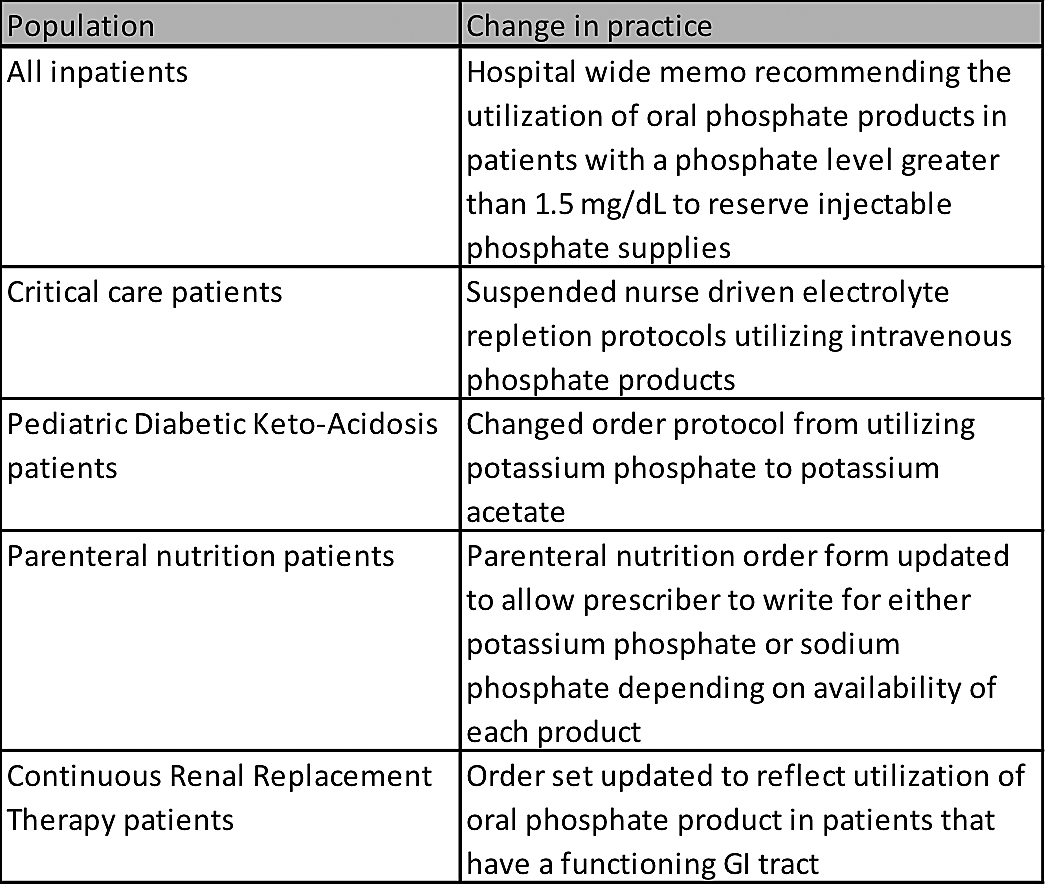
Table 1. Critical Drug Shortage Taskforce Actions to Conserve Injectable Phosphate
Utilization of injectable phosphate products decreased by more than than 50%, allowing the pharmacy to fulfill any patient orders that met defined guidelines. Prescribers who wrote for injectable phosphate product outside of these criteria were contacted by the pharmacy to recommend oral phosphate repletion. All adult intravenous phosphate orders were also ordered in standard dose increments to reduce waste and allow for redispensing if a dose was returned to the pharmacy with good dating.
Conclusion
The cases described above are emblematic of the critical nature of drug shortages occurring in the U.S. market impacting healthcare systems. In many instances, owing to unlimited access to drugs, agents were used liberally with little oversight and stewardship. These shortages have forced this discipline to occur, which actually has resulted in savings and improved quality of care, In other instances, ranging from oncology, where poorer outcomes have resulted, to critical care where paients are put in jeopardy due to the unavailability of critical electrolyes, harm has/could occur. Third, even small changes in packaging and vehicle can create safety concerns in critical situations, where routine is important. Lastly, increased labor costs and high-cost substitutes, strain an already expensive healthcare system. It is vital that the pharmacy work closely with physicians and nurses to collaborate in decisions regarding alternative concentrations, vehicles and substitutions, as well as education of front-line staff to avoid administration errors, which these changes engender.
Hospital leadership must ensure there is a structured approach to escalate drug shortages that carry a significant risk of interrupted supply affecting the quality of care delivered to patients. The structure we set up provided a highly efficient, safe, and effective way of managing these critical shortages, which allowed for the most effective use of key pharmacy and hospital resources. Our group recommends other organizations that have not yet organized a high-level committee involving quality and patient safety, risk management, pharmacy, and physician leadership to work on forming a similar multidisciplinary team.
All of the authors work at UMass Memorial Healthcare. Neil Gilchrist is manager of pharmacy operations and may be contacted at neil.gilchrist@umassmemorial.org. Fran Bassett is supervisor of IV/OR pharmacy services. Roland Bercume is senior director of pharmacy services. Denis Brown is manager of revenue and quality. Although he no longer works at UMass Memorial Healthcare, Robert Klugman was, at the time this article was written, associate professor of medicine and quantitative health services and senior vice president, chief quality officer, and medical director of managed care. He is currently vice president of medical affairs for the Eastern Region, Kindred Healthcare.
REFERENCE
Fox, E. R., Birt, A., James, K. B., Kokko, H., Salverson, S. & Soflin, D. L. (2009). ASHP guidelines on managing drug product shortages in hospitals and health systems. American Journal of Health-System Pharmacists, 66, 1399-1406.
Metzger, M. L., Billett, A. & Link, M. P. (2012, December 27). The impact of drug shortages on children with cancer—The example of mechlorethamine. New England Journal of Medicine, 367(26), 2461-2463.
Staff Report U.S. House of Representatives. (2012, June 15). FDA’s contribution to the drug shortage crisis. 1-21.
What Do Drug Shortages Mean for Patient Safety? |
By Christian Hartman, PharmD, MBA, FSMSOIn recent years, the United States has experienced an increasing number of drug shortages, which frustrate to clinicians and potentially compromise safe care of patients. Drug shortages affect the timeliness of drug preparation and dispensing and influence the use of alternative agents. Common reasons for drug shortages include business decisions made by manufacturers to decrease production, mergers, scarcity of raw materials, Federal Drug Administration (FDA) enforcement actions, natural disasters, and poor inventory management. Drug shortages force healthcare providers to use alternative agents, which potentially present increased risk to patients due to misuse or inferior efficacy compared to the intended agent. A national survey conducted in 2010 revealed that patient safety concerns have resulted from widespread drug shortages across several drug classes (ISMP). Of the 1,800 healthcare practitioners surveyed, 85% note that there is little or no information available about the duration of the drug shortage, 70% lack suitable alternative products, and 64% believe drug shortages increase the risk of adverse patient outcomes. Further, 35% of respondents reported a near miss, 25% reported a medication error, and 20% reported adverse drug events (ADEs), all associated with drug shortages. Management of drug shortages presents a significant challenge for healthcare organizations and places patients at higher risk for medication-related errors. This increased risk to patient safety has caused professional associations and the FDA to place significant emphasis on the safe management of drug shortages. On July 9, 2012, President Obama signed into the law the Food and Drug Administration and Innovation Act (FDASIA) of 2012. The FDASIA provides the FDA with the authority to expedite inspections, work with manufacturers willing to increase production, permit temporary foreign importation, and requires the FDA to provide an up-to-date list of drugs in shortage. The new FDA actions have been noted to improve certain drug shortage situations but has not addressed all concerns that exist today. With drug shortages becoming the “new norm,” healthcare organizations must have a comprehensive drug shortage management program to prevent patient harm and maintain high quality care. Chris Hartman is founder and Advisory Board chair of the Medication Safety Officers Society and senior director of clinical quality and patient safety for Wolters Kluwer Health. |
REFERENCEInstitute for Safe Medication Practices. (2010, July 29). Drug shortages threaten patient safety. ISMP Medication Safety Alert! Available at http://www.ismp.org/newsletters/acutecare/articles/20100729.asp Institute for Safe Medication Practices. (2010, September 23). Drug shortages: National survey reveals high level of frustration, low level of safety. ISMP Medication Safety Alert! Available at http://www.ismp.org/newsletters/acutecare/articles/20100923.asp |
