Data Trends: High-Alert Medications: Error Prevalence and Severity
July / August 2009
![]()
Data Trends
High-Alert Medications: Error Prevalence and Severity
Use of medications is the most common patient treatment intervention in healthcare. It is also the most common source of adverse events in the inpatient setting (Leape et al., 1991). Adverse events from medication usage increase morbidity and mortality as well as the overall cost of care. Based on a rate of 400,000 adverse drug events per year in hospitalized patients, the Institute of Medicine (IOM) Committee estimated that adverse drug events (ADEs) accounted for $3.5 billion (in 2006 dollars) of additional hospital incurred costs (Institute of Medicine, 2007).
Medication errors are defined by the National Coordinating Council for Medication Error Reporting and Prevention (NCC MERP) as:
Any preventable event that may cause or lead to inappropriate medication use or patient harm while the medication is in the control of the healthcare professional, patient, or consumer. Such events may be related to professional practice, healthcare practice, healthcare products, procedures, and systems, including prescribing; order communication; product labeling, packaging, and nomenclature; compounding; dispensing; distribution; administration; education; monitoring and use (n.d.).
All medications used improperly can have an adverse impact on patients, but a subset of drugs has increased potential for significant patient harm due to errors. These medications are commonly known as high-alert medications, a term coined by the Institute of Safe Medication Practices (ISMP). ISMP periodically provides healthcare providers with an updated list of higher-risk medications based on event reports submitted to the MEDMARX National Data Repository and the ISMP Medication Error Reporting program, as well as using medication adverse-event literature and input from clinicians and safety experts.
Many high-alert medications have a high volume of use. Examples of high-usage medications include insulin amongst diabetics, anticoagulants, narcotics, and sodium chloride for injection. Using any of these drugs increases the likelihood that a patient might suffer adverse events. Adverse events from these medications represent areas of greatest harm and greatest opportunity improving patient safety. Harm is defined by NCC MERP (2001) as “Impairment of the physical, emotional, or psychological function or structure of the body and/or pain resulting there from.”
There are many types of medication errors: wrong drug, wrong dose, wrong route or time of administration, or wrong patient. Flynn (2003) reported the percent of these errors in the outpatient pharmacy setting, with wrong label instructions at 52%, the highest reason for medication errors, followed by wrong label information, wrong quantity and strength. In addition, clinicians often fail to recognize or account for drug-drug interactions, drug-disease interactions, drug-food interactions, drug-lab issues, and drug allergies. Modern drug therapy is complex—much can go wrong, and it often does.
One of the 12 interventions that Institute for Healthcare Improvement (IHI) recommends for its 5 Million Lives Campaign is “Prevent harm from high-alert medications, starting with a focus on anticoagulants, sedatives, narcotics and insulin. (McCannon, Hackbarth, & Griffin, 2007)”
MEDMARX (a nationally recognized, web-based, anonymous, and voluntary medication error reporting system used by various types of healthcare facilities to report medication error data) was the source for the findings presented in this analysis. MEDMARX provides event reporting with analytical capabilities, including de-identified external comparative reports. The healthcare organization profile is used to stratify data according to various comparison criteria to create an environment where like-facility data can be compared. MEDMARX has adopted the medication error definition from NCC MERP.
Data in MEDMARX have been analyzed in various ways, including publication and review of annual reports. Writers in a recent publication that focused on the value of voluntary medication error reporting systems and analysis of warfarin-related medication errors, have opined that voluntary reporting systems can, to some extent, provide meaningful and actionable information to guide safety initiatives, but suffer from limitations due to underreporting and other biases (Zahn et al., 2008). This analysis focuses on identifying various attributes of medication errors due to many of the medications listed in ISMP’s 2008 High-Alert Medication List.
The following list enumerates the drugs used for this analysis.
- Alteplase, recombinant
- Colchicine
- Digoxin
- Epoprostenol
- Heparin
- Insulin, all types
- Magnesium sulfate
- Methotrexate
- Milrinone
- Nitroprusside
- Opium tincture
- Oxytocin
- Potassium chloride for injection concentration
- Potassium phosphates
- Promethazine
- Sodium chloride, hypertonic
- Sterile water for injection
- Sterile water for irrigation
- Tenecteplase
- Warfarin
Results of Analysis:
From 2006 through 2008 (event dates within last three years), 443,683 medication errors were reported from approximately 537 facilities, of which 98% were reported from an inpatient setting. Of these, 32,546, or 7%, were related to the high-alert medications listed above. There was no significant year-to-year variation in the number of reports submitted to MEDMARX.
While the majority, 54%, were located in inpatient care units, 21% were reported in the inpatient pharmacy departments, and 7% were reported in all ICUs combined.
Age distribution for these high-alert medication errors show that the highest proportion (29%) occurred in the 45-64 year age group, followed by the 65-74 and 75-84 year age groups, equally at 23%. A notably small proportion of ADEs occurred in the neonate category, children and 1-17 year age groups, that may be attributable to the non-use (not indicated in pediatric population) of all the high-alert medications (except heparin flush) for these age groups. Also the limitation of not having denominator data, makes the analysis difficult to calculate rate-based indicators for assessing propensity of such ADEs by age groups. However, we find that ADEs were higher in the 45-64 age group, contrary to current expectations, perhaps because of the high percentage of insulin usage in this population group.
In our analysis, the most commonly reported drug from the high-alert medication list was insulin, which was attributed to 45% of the errors, followed by heparin and warfarin, each at 21%.
The severity classification used in MEDMARX follows the NCC MERP taxonomy (2001) of medication error patient outcome categories. The severity for the events is categorized as follows: A: no error; B–D: error, no harm, E–H: error, harm; and I: error, death. The distribution of harm associated with the high-alert medication errors is shown in Figure 1, with the majority falling in the B–D categories (93.14%). While the majority of these High-Alert Medication errors resulted in no harm, a substantial number had harm associated with them and category D (23%) required monitoring/intervention to prevent harm.
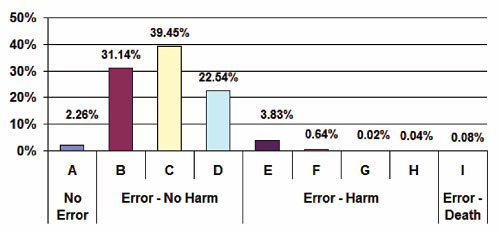
Figure 1. High-Alert Medication Errors by Harm Category 2006–2008
Of those ADEs where harm occurred, the majority resulted from insulin, heparin, and warfarin, following the frequency of use and error curves. Digoxin and promethazine were also associated with harm (Figure 2).
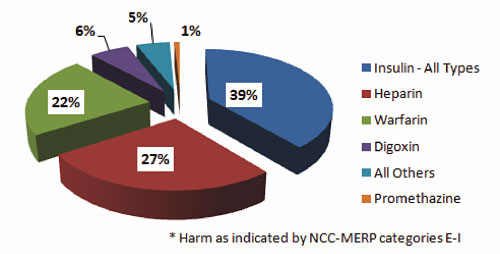
Figure 2. High-Alert Medication Errors for Drugs with Harm 2006–2008
The types and distribution of errors in MEDMARX is shown in Table 1, and the analysis shows that “omission error” and “improper dose/quantity” were the most frequent types of errors reported in the database. Notably, “unauthorized/wrong drug” came in third at 17%.
| Type of error category | Number | Percent |
| Omission error | 8,461 | 26% |
| Improper dose/quantity | 7,124 | 22% |
| Unauthorized/wrong drug | 5,463 | 17% |
| Prescribing error | 2,923 | 9% |
| Wrong time | 2,300 | 7% |
| Extra dose | 2,256 | 7% |
| Wrong patient | 1,786 | 5% |
| Mislabeling | 646 | 2% |
| Wrong dosage form | 586 | 2% |
| Wrong administration technique | 335 | 1% |
| Drug prepared incorrectly | 311 | 1% |
| Wrong route | 252 | 1% |
| Other | 113 | 0% |
| Total | 32,556 | 100% |
Figure 3 shows the distribution of the processes where the medication errors were initiated, with the highest reported instances taking place during the administering and dispensing processes (29% each), followed by the transcribing/documenting process at 25%.
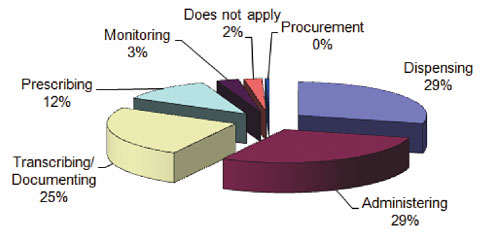
Figure 3. High-Alert Medication Errors by Administration Process Node 2006–2008
This analysis indicates that these most commonly involved processes deserve special attention when a process redesign intervention is used to reduce errors in medication delivery systems throughout various healthcare settings.
In MEDMARX, causes and contributory factors are categorized separately, although semantically these bear similar concepts. When highlighting the factor that was casually associated with any ADEs, the term “causal factor” is not necessarily used as a rigorous scientific expression. For both these factor types, multiple entries are allowed to be indicated for each event reported, so the dataset contains a rich set of variables associated with the events. While not reported here in depth, of note, “human performance deficit” was identified as the most common self reported causal factor (19%), while staffing issues were the highest reported contributory factor (31%) associated with ADEs.
Discussion and Summary
Reviewing data such as the prevalence, event severity, and contributory/causal factors for errors can help organizations examine process issues and enact interventions for improvement. Many factors contribute to care environments that are not conducive to patient safety, and various interventions must be implemented and monitored to ensure optimal outcomes.
The National Center for Patient Safety (NCPS) of the U. S. Department of Veterans Affairs has identified a number of practices, some of which are detailed in the sidebar. Medication errors can be can be significantly reduced by understanding the prevalence, care processes, and contributing factors of errors, followed by changes in practice. Based on these analyses, implementation of proven safety practices such as limiting access to such medications, using auxiliary labels, dosing on unit basis, having redundant checking procedures, standardizing prescribing rules, and creating an environment that is not burdensome for the care providers and fosters professional excellence can be undertaken.
Adverse drug events result in significant human and financial burdens. Voluntary reporting systems can provide valuable insights into the highest priority areas on which organizations should focus attention to reduce and monitor such errors.
All of the authors work for Quantros, Inc., a company based in Milpitas, California, that provides more than 2,000 healthcare facilities with real-time solutions for safety and risk management, outcomes and performance monitoring, accreditation and compliance, surveillance, and decision support. The MEDMARX application used by healthcare facilities is operated and managed by Quantros, Inc. Ali Rashidee is director of the Quantros SRM Product Suite, Juliana Hart is clinical marketing manager, Jack Chen is a biostatistician, and Sanjaya Kumar is chief medical officer. Dr. Rashidee may be contacted at arashidee@quantros.com.
References
Cohen, M. R. (Ed.) (2007). Medication error (2nd ed). Washington, DC: American Pharmacists Association.
Flynn, E. A., Barker, K. N., & Carnahan, B. J. (2003). National observational study of prescription dispensing accuracy and safety in 50 pharmacies. Journal of the American Pharmacists Association, 43(2), 191-200.
Institute for Safe Medication Practices. (2008). ISMP’s list of high-alert medications. Available at http://www.ismp.org/Tools/highalertmedications.pdf
Institute of Medicine. Committee on Identifying and Preventing Medicaiton Errors. Board on Health Care Services. (2007). Preventing medication errors. Quality Chasm series. P. Aspden, J. A. Wolcott, J. L. Bootman, & L. R. Cronenwett (Eds.). Washington, DC: National Academies Press.
Leape, L. L., Brennan, T. A., Laird, N., Lawthers, A. G., Localio, A. R., Barens, B. A, Herbert, L., et al. (1991, February 7). The nature of adverse events in hospitalized patients. Results of the Harvard Medical Practice Study II. New England Journal of Medicine, 323, 377-384.
McCannon, C. J., Hackbarth, A. D., & Griffin, F. A. (2007). Miles to go: An introduction to the 5 Million Lives Campaign. Joint Commission Journal on Quality and Patient Safety, 33(8), 477-484.
The National Coordinating Council for Medication Error Reporting and Prevention (NCC MERP). (n.d.). What is a medication error? Available at http://www.nccmerp.org/aboutMedErrors.html
The National Coordinating Council for Medication Error Reporting and Prevention (NCC MERP). (2001). NCC MRP index for categorizing medication errors. Available at http://www.nccmerp.org/pdf/indexColor2001-06-12.pdf
U. S. Department of Veterans Affairs. National Center for Patient Safety. High-Alert Medications. Available at http://www.va.gov/NCPS/NEWS/NCPSBg/highalertmeds.html
Zhan, C., Smith, S. R., Keyes, M. A., Hicks, R. W., Cousins, D. D., & Clancy, C. M. (2008). How useful are voluntary medication error reports? The case of warfarin-related medication errors. Joint Commission Journal on Quality and Patient Safety, 34(1), 36-45.
