Barcoding and RFID: Barcoding to Enhance Patient Safety
September / October 2005
Barcoding and RFID
Barcoding to Enhance Patient Safety
The National Coordinating Council for Medication Error Reporting and Prevention (NCCMERP) defines a medication error as “…any preventable event that may cause or lead to inappropriate medication use or patient harm while the medication is in the control of the healthcare professional, patient, or consumer” (2004). By definition, these errors can occur at any stage in the medication process, which includes prescribing, order communication, product labeling, packaging, compounding, dispensing, distribution, and administration. Various information technologies have been applied to reduce errors throughout the medication process (Chung et al., 2003; Oren et al., 2003; Bates, 2000), including computerized prescriber order entry (CPOE), robotics, automated dispensing devices, and the electronic medication administration record (eMAR). This article focuses on barcode technologies applied at the administration stage of the process.
Research has shown that errors that occur earlier in the medication process are more readily detected (~50% are prevented during the ordering stage) while very few (< 2%) are caught at the administration stage (bates et al., 1995). further, it has been noted that more than one third of medication errors occur at the latter stage (leape et al., 1995). because of the relatively high proportion of errors and the lack of success preventing them, error reduction strategies targeted at the administration stage have recently received significant attention from providers, specialty societies, and regulatory agencies.
An emerging information technology designed to address medication administration errors uses barcodes on both the medication and patient that are scanned at the point-of-care (POC) before administration. At its most basic level, barcode-assisted prevention of medication administration errors is predicated on verifying the “5 rights”; the right patient, right drug, right dose, right route, and right time.
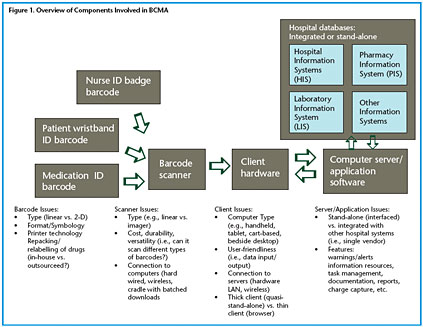
A summary of the barcode-assisted process for medication administration is shown in Figure 1. As can be seen, the barcoded medication administration (BCMA) technology is comprised of a number of hardware and software components that may include a barcode printing system for both wristbands and medication packages in the pharmacy; portable barcode scanners interfaced to computers located near the patient; a communications network (usually wireless) tying the portable bedside computers to a centralized computer server; software applications running on the server that process the incoming information and return information to the bedside computers; and interfaces for communication between the server/software and other hospital databases, such as the pharmacy information system and the admission discharge transfer (ADT) system.
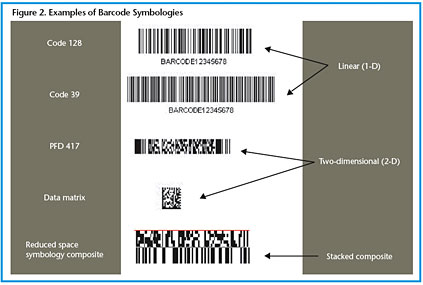
A typical procedure starts at the bedside with nurses scanning their own employee ID barcode, a barcode on the patient wristband, and a barcode on each medication package. The barcodes may be linear (1-D), two-dimensional (2-D), or composite (combined 1-D and 2-D elements) (HIBCC 2005, UCC 2005). More than one type of barcode may be involved in the medication administration process (see Figure 2). At this time, there are many different codes and symbologies that are not yet standardized, and each has advantages and disadvantages. Linear barcodes are the most common; for medications they typically contain the 10-digit National Drug Code (NDC) number that identifies the manufacturer, product, and package size. However, a hospital may use its own smaller, unique code to point to a much larger descriptor of the coded item contained in a relational database. Alternatively, the 2-D symbologies can contain more information, such as the lot number and expiration date of the product.
Technology Status and Significance
High rates of preventable medication errors have been repeatedly reported in studies in the medical literature (Bates et al., 1995; Leape et al., 1995; Flynn et al., 2002; Kanjanarat et al., 2003). It is difficult, however, to cite a single number to define the extent of the medication error problem due to differences in institutions, study methodologies, error definitions, and other variables. On the high end of estimates, one study that compiled data from 36 institutions reported 19% (~1 in 5) of the medication doses studied over a 4-day period involved medication errors (Barker et al., 2002). These errors included wrong time (43%), omission (30%), wrong dose (17%), and unauthorized drug (4%). The number of these errors deemed potentially harmful adverse drug events (ADEs) was 7%. A comprehensive review of medication error studies cited in the Institute of Medicine (IOM) 2000 report on errors in the U.S. healthcare system suggests that preventable ADEs, i.e., harmful medication errors, occur in ~1% to 10% of hospital admissions. The IOM report further estimated that 770,000 patients are injured and ~7,000 die each year due to medication errors.
However, not all ADEs are preventable, not all medication errors result in ADEs, and not all ADEs are serious. An estimated 28% to 95% of ADEs can be prevented (AHCPR, 2004). An allergic reaction to a drug in a patient with no previously known allergies is an example of a non-preventable ADE. Furthermore, missing a medication dose or providing the dose late may result in no discernible morbidity. On the other hand, error events involving cardiovascular drugs can result in serious complications secondary to bradycardia or hypotension, errors involving central nervous system (CNS)-acting agents can result in serious complications from oversedation and respiratory depression, errors involving anticoagulants may result in bleeding episodes, errors involving insulin can lead to a hypoglycemic episode, and errors involving chemotherapy agents can lead to bone marrow depression and toxicity (Kanjanarat et al., 2003).
Fortunately, the rate of serious injury and death stemming from medication errors is low. One study suggested ~12% of ADEs were serious and ~1% were fatal (Bates et al., 1995). Another study suggested that ~6.7% of hospitalized patients experienced ADEs, with 0.32% of those being fatal (Lazarou et al., 1998). However, even if one determines the rate of medication administration errors resulting in significant injury or death is very small, it remains a significant problem owing to the high number of medication administrations that occur annually. The IOM report estimated that 3.75 billion drug administrations were given to patients in hospitals in 1998.
The added costs associated with treating medication errors can be very high (Classen et al., 1997; Bates et al., 1997). For example, one study found that ~2% of admissions experienced a preventable ADE with an added cost per patient of ~$4,700 (JCAHO, 2004). Extrapolated, this suggests an added cost of ~$2.8 million per year for a 700-bed teaching hospital. This figure does not include the significant costs of defending against malpractice claims stemming from preventable inpatient ADEs (Rothschild et al., 2002).
The push to use barcodes to improve medication administration safety has come from many organizations, including professional societies, hospital networks, industry consortiums, and patient safety groups. In early 2004, it appeared that the Joint Commission on Accreditation of Healthcare Organizations (Joint Commission) would mandate that hospitals adopt the technology via implementation of Joint Commission National Patient Safety Goals (NPSGs) for 2005 (JCAHO, 2004). However, final wording of the NPSGs, approved in July 2004, did not specifically mention medication barcoding. One reason for the omission included the inability to determine a time frame for which it would be reasonable for hospitals to reach the standard.
The adoption of barcode technology by hospitals has been slow. The first prototype systems were developed in the early to mid-1990s and began to be disseminated in the late 1990s. According to the American Society of Health-System Pharmacists (ASHP) national survey of pharmacy practice, 1.1% of hospitals used barcode technology for medication administration in 1999; a re-survey conducted in 2002 reported a small increase to 1.5% of hospitals (Pedersen et al., 2003). A manufacturer’s estimate in the summer of 2003 suggested that about 300 of the nation’s 6,600 hospitals had BCMA systems (130 of which were Veterans Administration [VA] hospitals. [Information Week, 2003]) Recently, with the entry of many more manufacturers and products in the marketplace, it is expected that the rate of implementation is climbing, though it is still probably in the range of 5% to 10% of hospitals.
Medication Error Data — UHC Patient Safety Net
Insight into the current status of medication errors at academic medical centers can be derived from the UHC-compiled Patient Safety NetTM (PSN). PSN is a real-time, Web-based adverse event reporting tool used by subscribing UHC members to report and evaluate patient safety and improve health care quality. In summer of 2004, 17 participating hospitals used PSN. The following analyses were excerpted from all events in PSN recorded between October 2002 and March 2004.
More than 13,000 events related to medication use were noted in PSN, 11,633 of which were reported as medication errors. Most of these errors (~91%) caused no harm to the patient. However, medication errors caused permanent harm or death in 0.3% (40/11,633) and resulted in temporary harm requiring intervention, treatment, and/or prolonged hospitalization in 5%.
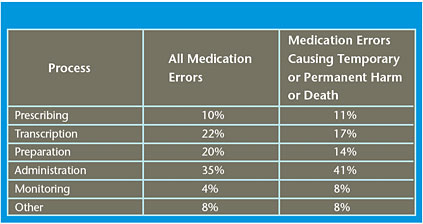
Reported medication errors are grouped according to the stage in the medication process in which they occurred (see Table 1). Similar to data reported in the literature, UHC data suggest administration errors account for ~35% of all medication errors and 41% of errors causing harm.
Administration errors are more likely to reach the patient than errors that occur earlier in the medication process. Further, when errors occur in the latter stages of the medication process (administration and monitoring) they result in a higher proportion of cases requiring further treatment, monitoring, and/or prolonged hospitalization.
Implementation Issues
Steps to be taken in the pre-implementation phase include a thorough study of the current medication administration system and processes to delineate problems and issues and develop criteria for functionality of the final product. Initial studies often serve to determine the baseline number and type of medication errors that occur to compare them with those that occur after implementation of the barcode system. A thorough study of the information system (IS) infrastructure is performed to determine current capabilities and map out future functionalities. IS upgrades should be carried out prior to implementing barcoding technologies. In this phase, a multidisciplinary committee is formed that consists of members from nursing, pharmacy, IT, and administration. This body meets regularly to discuss all stakeholder views. Successful implementation may depend on the input provided early on from this committee, particularly input from end users.
In this pre-implementation phase, some institutions may choose to stage implementation of barcoding by first successfully barcoding patient IDs, followed by staff ID barcoding. Next, arrangements must be made regarding barcoding processes for medications. There are a number of models, including outsourcing medication barcodes, using in-house repackaging with barcoding, relying on manufacturer pre-barcoding of unit dose packages, or employing a combination of these methods. Barcoding of medications is a complicated logistical problem that will require significant pharmacy space, time, and effort.
In addition, a pilot application is typically recommended. This is performed on a single unit and serves to test the technology at all levels. Only after a successful pilot is functional for a specific period of time should the technology be rolled out on a larger scale.
Selecting a product is a complicated process that is very institution-specific. The involvement of end users in equipment selection is very important for obtaining cooperation and choosing important capabilities. A key generalized capability should be the ability of the system to provide information to the caregiver at the point of administration. This information includes drug/drug interactions, instructions for administering medications, decision support materials, and prompts for further information, such as taking blood pressure prior to administration. It was also suggested that the ability to customize the type of information provided was very important; one example is the ability to turn off certain prompts or warnings that are deemed unnecessary or that are impeding workflow efficiency with little gain in patient safety.
Despite the general acceptance that BCMA systems are valuable for patient safety, their use still has challenges, potential disadvantages, and may even introduce different types of errors. Some of these may include:
- Errors can occur in the printed barcode label (patient or drug).

- Not all drugs will contain manufacturer barcodes, and not all manufacturer codes are standardized and/or map to customized hospital pharmacy formulary databases.

- Relabelling and repackaging drugs have associated costs and can introduce errors.

- Procedures may contain loopholes that enable users to circumvent key steps, thus negating error safeguards.

- Interfacing between various hospital computer systems can be problematic.

- Barcode scanning can interrupt nurse workflow processes, leading to frustration and fatigue among staff.

- Barcode equipment must be reliable, readily available, and user-friendly to be used effectively.

- Select barcode technologies may lack certain desirable features/functions, which limit their usefulness.

- Barcode system approaches to complex IV solutions, nontypical formulations, and other pharmacy compounded products are still in the development phase.
Future Developments
A BCMA system is only one part of a comprehensive information technology-enabled medication system. The complete system of the future will also incorporate CPOE, eMARs, and smart pumps to ensure patient safety from different aspects of the medication process.
Radio frequency identification (RFID) tags have been considered as future replacements for the barcodes now placed on wristbands, staff IDs, and drug packages. This technology has recently gained popularity in select applications, mainly inventory management. Pilot programs typically call for RFID labeling of bulk packages or pallets with the ultimate goal of individual package labeling. Further, one manufacturer of automated drug dispensing cabinets has introduced a cabinet that keeps track of RFID labeled packages sitting within its shelves. In another example of disseminating RFID technology, drug manufacturers have RFID tagged select drug packages, such as oxycontin and Viagra, in an effort to thwart counterfeiting schemes.
The applications most likely to use RFID tags at an early date are labeling of blood products and IV bags. In these cases, the cost of the product is much greater than the label cost, and there are some difficulties affixing a barcode to the shape of the product bag; these bags typically require a large amount of information beyond what the NDC code provides, and thus sometimes requires multiple, long barcodes that can be technically difficult to place and cumbersome to read.
The technology for RFID tags and RFID readers continues to mature. The common perception is that the technology may be ready for POC applications in 3 to 5 years. It is likely that most institutions planning medication administration safety initiatives will first use barcodes rather than RFID tags. Since these safety initiatives involve a paradigm change in administration processes, this tends to transcend any one technology involved in the process; thus, it is recommended that institutions begin their implementation without waiting for RFID technology. If RFID matures to the point of viability, it may be possible at some future date to substitute RFID tags for barcodes and RFID scanners for barcode scanners with little interruption to the process.
Barcode technologies are now being used for other healthcare applications outside of medication administration. Probably the other most evolved and important application involves safety initiatives that target lab specimen identification. This involves patient identification at the POC (by reading a wristband barcode), obtaining the sample, and immediately identifying it with a portable printer-based label. The label also contains a barcode for inputting the specimen data at the hospital lab. Most BCMA manufacturers also have, or are planning to have, a module for lab specimen identification. Similarly, some manufacturers began with the lab application as their main product and are now branching into the medication administration application.
Barcodes may also play an expanded role in the trend toward having a complete electronic health record (EHR). Barcodes are a highly accurate, convenient, and quick method of data entry. Possible future applications involve all aspects of the care process whereby the nurse may scan a barcode from a master sheet for any services/actions performed, thus electronically entering the action into the EHR. Similarly, barcodes may be involved in dietary management, from ordering to billing. Numerous other applications can be envisioned where information that should be in the patient record is input by staff via a barcode scanner.
Acknowledgements
Kim Barker; Susan Bonini, MSN; Paul Bush, PharmD; William Churchill; Ralph Farr; Harold Godwin, MS; Judy Holbein; Patrice Jones, MSN; Brad Ludwig, MS; Garret Newkirk, PharmD; Lisa Swiontek; Julie Racioppi, MSN; Steven Rough, MS; Doug Smith, PharmD; and Linda Stencel.
Joseph Cummings prepares resources created by the technology assessment group. Since joining UHC in 1994, he has authored or contributed to more than 50 assessments and reports primarily involving medical devices, surgical procedures, diagnostic lab tests, and transfusion medicine technologies. Cummings’ background includes a bachelor’s degree in biomedical engineering from the University of Iowa and a doctorate in biomedical engineering from Northwestern University.
Thomas Ratkomanages and prepares resources created by the technology assessment (TA) group, including High-Impact Technology Briefs, Web conferences, and other TA-related activities. Prior to joining UHC in 1993, Ratko was a senior research associate for the American Medical Association (AMA) in the Department of Immunology and Infectious Diseases. Tom received his doctorate in pharmacology degree from the University of Illinois Health Sciences Center in Chicago, and his bachelor of science degree in microbiology from the University of Illinois Urbana-Champaign.
Karl Matuszewski (matuszewski@uhc.edu) directs UHC’s Clinical Knowledge Service, a program of the Clinical Practice Advancement Center (CPAC). He is responsible for the development and direction of all UHC technology assessment efforts. Matuszewski received his bachelor of science and doctor of pharmacy degrees from the University of Illinois, where he is currently an assistant clinical professor, and a master of science degree in health systems management from Rush University, where he holds a faculty appointment as an associate professor. He is also an affiliate clinical assistant professor of pharmacy practice at Midwestern University.
References
AHCPR Report: Reducing and preventing adverse drug events to decrease hospital costs. Available at: www.ahcpr.gov/qual/aderia/aderia.htm. Accessed August 2004.
Barker, K. N., Flynn, E. A., Pepper, G. A., Bates, D. W., Mikeal, R. L. (2002). Medication errors observed in 36 health care facilities. Archives of Internal Medicine162(16), 1897-1903.
Bates DW. Using information technology to reduce rates of medication errors in hospitals. British Medical Journal320(7237):788-791.
Bates, D. W., Cullen, D. J., Laird, N., et al. Incidence of adverse drug events and potential adverse drug events. Implications for prevention. ADE Prevention Study Group. Journal of the American Medical Association274(1), 29-34.
Bates, D. W., Spell, N., Cullen, D. J., et al. The costs of adverse drug events in hospitalized patients. Adverse Drug Events Prevention Study Group. Journal of the American Medical Association277(4), 307-311.
Chung, K., Choi, Y. B., Moon, S. (2003). Toward efficient medication error reduction: error-reducing information management systems. Journal of Medical Systems27(6), 553-560.
Classen, D. C., Pestotnik, S. L., Evans, R. S., Lloyd, J. F., & Burke, J. P. (1997). Adverse drug events in hospitalized patients. Excess length of stay, extra costs, and attributable mortality. Journal of the American Medical Association277(4), 301-306.
Flynn, E. A., Barker, K. N., Pepper, G. A., Bates, D. W., & Mikeal, R. L. (2002). Comparison of methods for detecting medication errors in 36 hospitals and skilled-nursing facilities. American Journal of Health System Pharmacists59(5), 436-446.
InformationWeek Web site: May 19, 2003. Available at: http://www.informationweek.com/story/
showArticle.jhtml?articleID=10000106&pgno=3. Accessed November 2004.
IOM Report (2000). To err is human: Building a saferhealth system. Available at: http://books.nap.edu/books/0309068371/html/index.html. Accessed August 2004.
JCAHO National Patient Safety Goals Web site. Available at: http://www.jcaho.org/accredited+organizations/patient+safety/npsg.htm. Accessed November 2004.
Kanjanarat, P., Winterstein, A. G., Johns, T. E., Hatton, R. C., Gonzalez-Rothi, R., & Segal, R. (2003). Nature of preventable adverse drug events in hospitals: A literature review. American Journal of Health System Pharmacists60(17), 1750-1759.
Lazarou, J., Pomeranz, B. H., & Corey, P. N. (1998). Incidence of adverse drug reactions in hospitalized patients: a meta-analysis of prospective studies. Journal of the American Medical Association279(15), 1200-1205.
Leape, L. L., Bates, D. W., Cullen, D. J., et al. Systems analysis of adverse drug events. ADE Prevention Study Group. Journal of the American Medical Association274 (1), 35-43.
NCCMERP Web site. Available at: http://www.nccmerp.org/aboutMedErrors.html. Accessed June 2004.
Oren, E., Shaffer, E. R., Guglielmo, B. J. (2003). Impact of emerging technologies on medication errors and adverse drug events. American Journal of Health System Pharmacists60(14), 1447-1458.
Pedersen, C. A., Schneider, P. J., Scheckelhoff, D. J. (2003). ASHP national survey of pharmacy practice in hospital settings: Dispensing and administration — 2002. American Journal of Health System Pharmacists60(1), 52-68.
Rothschild, J. M., Federico, F. A., Gandhi, T. K., Kaushal, R., Williams, D. H., & Bates, D. W. (2002). Analysis of medication-related malpractice claims: causes, preventability, and costs. Archives of Internl Medicine162(21), 2414-2420.
The Health Industry Business Communications Council® (HIBCC®) Web site. Available at: http://www.hibcc.org/about.htm. Accessed January 2005.
The Uniform Code Council (UCC) Web site. Available at: http://www.uc-council.org. Accessed January 2005.
- SPECIAL SECTION:
Barcoding and RFID

- • Automatic Identification

- • Intelligent Location

- • Labeling and Tracking Preventing Errors in the Lab

- • Barcoding/RFID Resource Directory